Family
The protein belongs to the phosphoglycerate mutase superfamily. The main characteristic of the proteins in this family is the presence of the PGAM functional domain. However, most of the PGAM proteins are responsible for the transference of the phosphate group in small molecules. The proteins of this family participate in the glycolysis process, more specifically, in the transference of the phosphate group of the compound 3-phosphoglycerate, forming 2-phosphoglycerate[1]. PGAM5 and the T-cell signaling protein suppressor proteins (STS-1 and STS-2) are the only human proteins in this family that have a known phosphatase function[1][2].
Structural Highlights
The vast majority of mitochondrial proteins have an N-terminal sequence that indicates that they must be exported to the mitochondria. This sequence is called the mitochondrial signal peptide. When the protein enters the mitochondria, this sequence is usually cleaved, providing greater stability. It is still unknown what this sequence would be in the case of PGAM5 since the protein signal peptide is not cleaved[3] and PGAM5 is anchored in its entirety to the inner membrane through its transmembrane domain, defined by amino acids 9-29[4].
Regarding the catalytic activity, is responsible for the nucleophilic attack of the phosphate of the target protein, performing the intermediate link between the protein and the phosphate. However, histidine 105 is part of the canonical RHGE motif, present in most proteins of the PGAM family, forming part of the PGAM domain (98-289)[4]. Protein is induced by the at amino acids 58-63 which has been shown to function as an allosteric regulator of the specific phosphatase activity of PGAM5[2]. Mutations in this motif prevent oligomerization of the enzyme but still present as since the (270-289) in PGAM5 is responsible for the dimerization of the protein[4]. These dimers, however, do not show phosphatase activity[2].
Function
Even though is a member of the PGAM protein family, it appears to lack phosphoglycerate mutase typical phosphotransferase and/or phosphohydrolase activities.[4] Instead, this protein is a serine/threonine (Ser/Thr) phosphatase, that is, it’s responsible for protein-protein interactions through dephosphorylation of serine/threonine and, occasionally, histidine residues[5][6]. Its active site is composed of a histidine residue (His-105) responsible for the nucleophilic attack of the phosphorus atom acting as a phospho-acceptor[4][6].
PGAM5 has been shown to interact with B-cell lymphoma-extra large (Bcl-Xl), an apoptosis regulator, indicating a probable regulating role in the apoptotic process.[1][7]. Moreover, it also acts as substrate for both the Kelch-like ECH-associated protein (Keap1) and Nuclear factor erythroid 2-related factor 2 (Nrf2), forming a ternary complex, located in the mitochondria, that regulate gene expression for Nrf2-dependent genes, that are antioxidants and provide protection from reactive oxygen species (ROS)[7][8].
Furthermore, it has been studied that cleaved PGAM5 plays an important role in regulation of mitophagy and mitochondrial fission, in cases in which the mitochondria has been damaged. In this instance, PGAM5 is released into the cytosol and can either stay in the cytosol or be translocated to the nucleus [9].
Additionally, it’s been suggested that PGAM5 can also be involved in cellular senescence, necroptosis, responses to stress, parasite replication, lipid metabolism, inflammatory and immune responses[9].
Subcelluar Localization
PGAM5 is a mitochondrial enzyme. However, the mitochondria is a complex organelle, composed of really distinct regions. It has 2 membranes, an outer membrane (OMM), in direct contact with the cytoplasm, and an inner membrane (IMM), in contact with the mitochondrial matrix. Between them there is the intermembrane space, where the protons from the matrix are directed Therefore, the mitochondrial matrix exhibits a higher pH than the intermembrane space.
For a long while, PGAM5’s subcellular localization in the mitochondria has been debated. It includes a transmembrane N-terminal domain, which indicates that it would be located in the IMM, facing the intermembrane space[9]. Nonetheless, it has been demonstrated that PGAM5 interacts with cytosolic proteins, indicating that it could not be located in the IMM, and should be, instead, located in the OMM, facing the outside of the mitochondria[1]. More recently, however, studies have shown that the entire protein is exported to the mitochondria, where it’s anchored in the IMM and, during the first stages of mitophagy, it’s cleaved by the PARL protease in the serine 24 residue and it’s then released in the cytosol, where it interacts with other proteins important to this process[3].
Evolutionary Conservation
As part of the PGAM protein family, it is expected that PGAM5’s sequence includes a histidine phosphatase superfamily domain typical to this set of proteins, ranging from amino acids 58 up to 287.
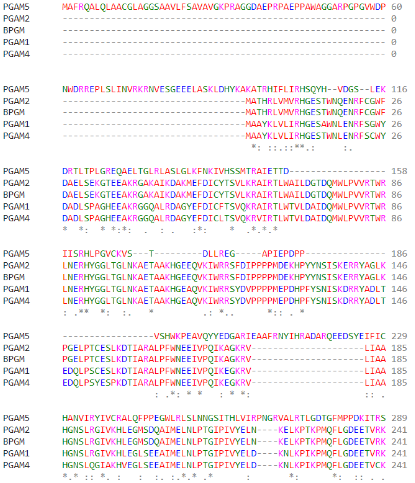
(Clustal Omega Multiple Alignment of different PGAM superfamily members)
Similarly, this same domain is also present in orthologs of PGAM5 in different species.
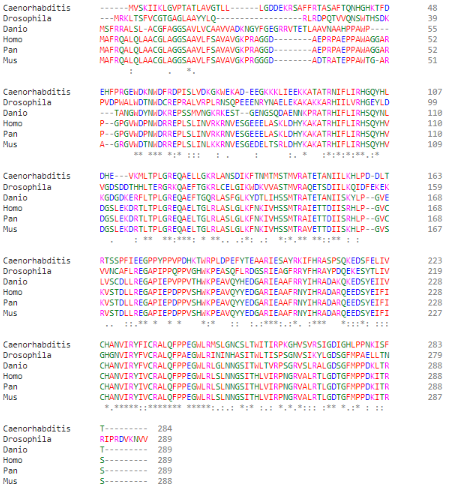
(Clustal Omega Multiple Alignment of PGAM5 in different species, them being: (top to bottom) C. elegans; D. melanogaster; D. rerio; H. sapiens; P. paniscus; M. musculus)
The conserved catalytic core contains an histidine residue within the active site (His-105) which is phosphorylated during the reaction. This specific residue is highly conserved through many members of the PGAM family and, moreover, through several different taxons.

(Highlight of the His-105 in different PGAM superfamily members)

(Highlight of the His-105 of PGAM5 in different species, them being: (top to bottom) C. elegans; D. melanogaster; D. rerio; H. sapiens; P. paniscus; M. musculus)
Related Diseases
It’s suggested that PGAM5 might possibly be associated with diseases caused by mitochondrial dysfunction, especially if it occurs in response to defective mitophagy process, including neurodegenerative diseases[10][11].
One such example is Parkinson’s disease, in which dopaminergic neurons die, likely due to oxidative stress and/or mitophagy defects. One of the proposed causes of Parkinson’s disease is a recessive mutation in autosomal gene responsible for the expression of PTENinduced putative kinase 1 (PINK1), a cytosolic and mitochondrial-associated kinase that regulates and promotes mitophagy in healthy cells, selectively eliminating dysfunctional mitochondria. This is mediated by accumulation of PINK1 in the mitochondrial outer membrane upon mitochondrial damage, which then recruits the E3 ubiquitin ligase Parkin, responsible for ubiquitinating MOM proteins and inducing degradation of mitochondria[10]. Furthermore, PGAM5 has also been reported as a regulator of PINK1/Parkin mediated mitophagy in mice, stabilizing said proteins, by binding to PINK1 and protecting it from proteases[11]. PGAM5 knockout (KO) mice have shown an almost total loss of mRNA nor protein expression, and these mice exhibited disrupted PINK1 stabilization and recruitment. Said mice were shown to have some, but not all, of the behavioral phenotype typical to Parkinson’s disease through dopaminergic neurodegeneration. It is therefore possible that deficiency in PGAM5 expression may be heavily associated with Parkinson’s disease and similar neurodegenerative conditions[11].
On the other hand, it is also possible that higher levels of PGAM5 may lead to diseases and complications. It has been studied that PGAM5 plays a role in non-small-cell lung cancer (NSCLC), albeit the mechanisms behind remain uncertain. It’s well documented that chronic obstructive pulmonary disease (COPD) patients have a higher risk of developing NSCLC, and that patients with NSCLC with established COPD have worse prognosis. Moreover, it is well recorded that oxidative stress contributes to the development of carcinogenic cells. In this sense, studies have reported that at least fourteen mitochondrial-related genes showed a fold difference in expression between non-cancerous and NSCLC cells, including PGAM5. This protein shows a large increase in not only mRNA expression but also protein levels in different types of lung cancer tissue compared to normal lung tissue. It’s still unknown how the overexpression of PGAM5 is related to lung cancer, though it is possible that the higher expression levels may lead to secondary uncontrolled cellular proliferation[12].
Autoimmune hepatitis (AIH) is an autoimmune disease that leads to acute liver injury caused by an immune-mediated necrosis of hepatocytes. It has been demonstrated that AIH patients show strong signs of mitochondrial aggregation and abnormalities, characteristics of mitochondrial dysfunction, as well as exhibit enhanced expression of PGAM5. It is also discussed that PGAM5 is a central mediator to the progression of the disease, as KO mice with blocked upstream regulators of PGAM5 do not appear to develop liver necrosis even in induced conditions. However, exactly how PGAM5 regulates acute liver necroinflammation remains elusive[13].
Additionally, it’s possible for PGAM5 to be associated with female infertility. Ovarian aging presents changes in the quantity and quality of oocyte reserves over time, which can cause fertility issues, especially age related. Oocyte senescence is correlated with higher oxidative stress and possible mitochondrial dysfunction, leading to energy insufficiency necessary for fertilization to occur. When studying several infertility patients, a positive correlation between age of cumulus and granulosa cells and the expression levels of PGAM5 and its co-regulators. Furthermore, the quality of the mitochondria is decreased in aged mice, and PGAM5 knockdown reduces the aging process. Similarly to AIH and NSCLC, overexpression of PGAM5 seems to disturb oocyte mitochondrial activity, and although it’s still not elucidated how this protein acts in this scenario, PGAM5 has enormous potential to be a diagnostic marker for possible infertility patients[14].




