| Structural highlights
Disease
A4_HUMAN Defects in APP are the cause of Alzheimer disease type 1 (AD1) [MIM:104300. AD1 is a familial early-onset form of Alzheimer disease. It can be associated with cerebral amyloid angiopathy. Alzheimer disease is a neurodegenerative disorder characterized by progressive dementia, loss of cognitive abilities, and deposition of fibrillar amyloid proteins as intraneuronal neurofibrillary tangles, extracellular amyloid plaques and vascular amyloid deposits. The major constituent of these plaques is the neurotoxic amyloid-beta-APP 40-42 peptide (s), derived proteolytically from the transmembrane precursor protein APP by sequential secretase processing. The cytotoxic C-terminal fragments (CTFs) and the caspase-cleaved products such as C31 derived from APP, are also implicated in neuronal death.[1] [2] [3] [4] [5] [6] [7] [8] [9] [10] [11] [12] [13] [14] [15] [16] [17] [18] [19] [20] [21] [22] [23] [24] [25] [26] Defects in APP are the cause of cerebral amyloid angiopathy APP-related (CAA-APP) [MIM:605714. A hereditary localized amyloidosis due to amyloid-beta A4 peptide(s) deposition in the cerebral vessels. The principal clinical characteristics are recurrent cerebral and cerebellar hemorrhages, recurrent strokes, cerebral ischemia, cerebral infarction, and progressive mental deterioration. Patients develop cerebral hemorrhage because of the severe cerebral amyloid angiopathy. Parenchymal amyloid deposits are rare and largely in the form of pre-amyloid lesions or diffuse plaque-like structures. They are Congo red negative and lack the dense amyloid cores commonly present in Alzheimer disease. Some affected individuals manifest progressive aphasic dementia, leukoencephalopathy, and occipital calcifications.[27] [28] [29] [30] [31]
Function
A4_HUMAN Functions as a cell surface receptor and performs physiological functions on the surface of neurons relevant to neurite growth, neuronal adhesion and axonogenesis. Involved in cell mobility and transcription regulation through protein-protein interactions. Can promote transcription activation through binding to APBB1-KAT5 and inhibits Notch signaling through interaction with Numb. Couples to apoptosis-inducing pathways such as those mediated by G(O) and JIP. Inhibits G(o) alpha ATPase activity (By similarity). Acts as a kinesin I membrane receptor, mediating the axonal transport of beta-secretase and presenilin 1. Involved in copper homeostasis/oxidative stress through copper ion reduction. In vitro, copper-metallated APP induces neuronal death directly or is potentiated through Cu(2+)-mediated low-density lipoprotein oxidation. Can regulate neurite outgrowth through binding to components of the extracellular matrix such as heparin and collagen I and IV. The splice isoforms that contain the BPTI domain possess protease inhibitor activity. Induces a AGER-dependent pathway that involves activation of p38 MAPK, resulting in internalization of amyloid-beta peptide and leading to mitochondrial dysfunction in cultured cortical neurons. Provides Cu(2+) ions for GPC1 which are required for release of nitric oxide (NO) and subsequent degradation of the heparan sulfate chains on GPC1.[32] [33] [34] [35] [36] Beta-amyloid peptides are lipophilic metal chelators with metal-reducing activity. Bind transient metals such as copper, zinc and iron. In vitro, can reduce Cu(2+) and Fe(3+) to Cu(+) and Fe(2+), respectively. Beta-amyloid 42 is a more effective reductant than beta-amyloid 40. Beta-amyloid peptides bind to lipoproteins and apolipoproteins E and J in the CSF and to HDL particles in plasma, inhibiting metal-catalyzed oxidation of lipoproteins. Beta-APP42 may activate mononuclear phagocytes in the brain and elicit inflammatory responses. Promotes both tau aggregation and TPK II-mediated phosphorylation. Interaction with Also bind GPC1 in lipid rafts.[37] [38] [39] [40] [41] Appicans elicit adhesion of neural cells to the extracellular matrix and may regulate neurite outgrowth in the brain (By similarity).[42] [43] [44] [45] [46] The gamma-CTF peptides as well as the caspase-cleaved peptides, including C31, are potent enhancers of neuronal apoptosis.[47] [48] [49] [50] [51] N-APP binds TNFRSF21 triggering caspase activation and degeneration of both neuronal cell bodies (via caspase-3) and axons (via caspase-6).[52] [53] [54] [55] [56]
Evolutionary Conservation
Check, as determined by ConSurfDB. You may read the explanation of the method and the full data available from ConSurf.
Publication Abstract from PubMed
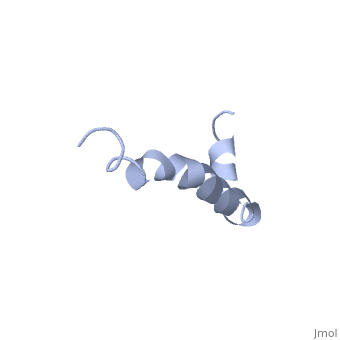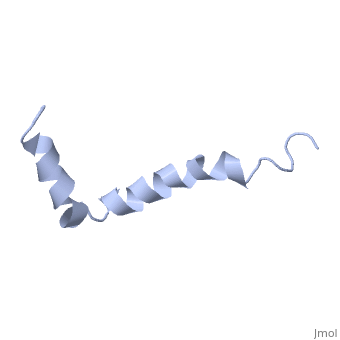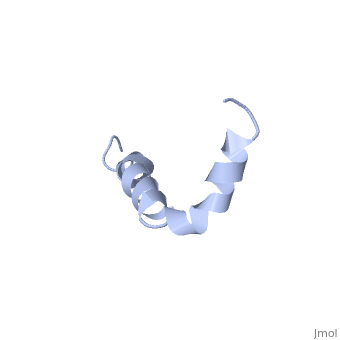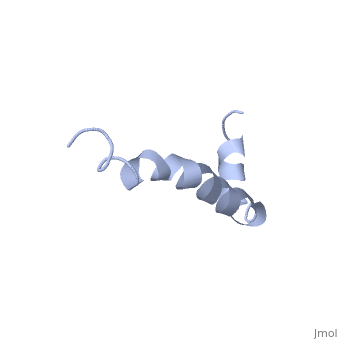
The major components of neuritic plaques found in Alzheimer disease (AD) are peptides known as amyloid beta-peptides (Abeta), which derive from the proteolitic cleavage of the amyloid precursor proteins. In vitro Abeta may undergo a conformational transition from a soluble form to aggregated, fibrillary beta-sheet structures, which seem to be neurotoxic. Alternatively, it has been suggested that an alpha-helical form can be involved in a process of membrane poration, which would then trigger cellular death. Conformational studies on these peptides in aqueous solution are complicated by their tendency to aggregate, and only recently NMR structures of Abeta-(1-40) and Abeta-(1-42) have been determined in aqueous trifluoroethanol or in SDS micelles. All these studies hint to the presence of two helical regions, connected through a flexible kink, but it proved difficult to determine the length and position of the helical stretches with accuracy and, most of all, to ascertain whether the kink region has a preferred conformation. In the search for a medium which could allow a more accurate structure determination, we performed an exhaustive solvent scan that showed a high propensity of Abeta-(1-42) to adopt helical conformations in aqueous solutions of fluorinated alcohols. The 3D NMR structure of Abeta-(1-42) shows two helical regions encompassing residues 8-25 and 28-38, connected by a regular type I beta-turn. The surprising similarity of this structure, as well as the sequence of the C-terminal moiety, with those of the fusion domain of influenza hemagglutinin suggests a direct mechanism of neurotoxicity.
Solution structure of the Alzheimer amyloid beta-peptide (1-42) in an apolar microenvironment. Similarity with a virus fusion domain.,Crescenzi O, Tomaselli S, Guerrini R, Salvadori S, D'Ursi AM, Temussi PA, Picone D Eur J Biochem. 2002 Nov;269(22):5642-8. PMID:12423364[57]
From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.
References
- ↑ Denman RB, Rosenzcwaig R, Miller DL. A system for studying the effect(s) of familial Alzheimer disease mutations on the processing of the beta-amyloid peptide precursor. Biochem Biophys Res Commun. 1993 Apr 15;192(1):96-103. PMID:8476439 doi:http://dx.doi.org/10.1006/bbrc.1993.1386
- ↑ Wakutani Y, Watanabe K, Adachi Y, Wada-Isoe K, Urakami K, Ninomiya H, Saido TC, Hashimoto T, Iwatsubo T, Nakashima K. Novel amyloid precursor protein gene missense mutation (D678N) in probable familial Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2004 Jul;75(7):1039-42. PMID:15201367
- ↑ Goate A, Chartier-Harlin MC, Mullan M, Brown J, Crawford F, Fidani L, Giuffra L, Haynes A, Irving N, James L, et al.. Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer's disease. Nature. 1991 Feb 21;349(6311):704-6. PMID:1671712 doi:http://dx.doi.org/10.1038/349704a0
- ↑ Yoshioka K, Miki T, Katsuya T, Ogihara T, Sakaki Y. The 717Val----Ile substitution in amyloid precursor protein is associated with familial Alzheimer's disease regardless of ethnic groups. Biochem Biophys Res Commun. 1991 Aug 15;178(3):1141-6. PMID:1908231
- ↑ Naruse S, Igarashi S, Kobayashi H, Aoki K, Inuzuka T, Kaneko K, Shimizu T, Iihara K, Kojima T, Miyatake T, et al.. Mis-sense mutation Val----Ile in exon 17 of amyloid precursor protein gene in Japanese familial Alzheimer's disease. Lancet. 1991 Apr 20;337(8747):978-9. PMID:1678058
- ↑ Chartier-Harlin MC, Crawford F, Houlden H, Warren A, Hughes D, Fidani L, Goate A, Rossor M, Roques P, Hardy J, et al.. Early-onset Alzheimer's disease caused by mutations at codon 717 of the beta-amyloid precursor protein gene. Nature. 1991 Oct 31;353(6347):844-6. PMID:1944558 doi:http://dx.doi.org/10.1038/353844a0
- ↑ Murrell J, Farlow M, Ghetti B, Benson MD. A mutation in the amyloid precursor protein associated with hereditary Alzheimer's disease. Science. 1991 Oct 4;254(5028):97-9. PMID:1925564
- ↑ Kamino K, Orr HT, Payami H, Wijsman EM, Alonso ME, Pulst SM, Anderson L, O'dahl S, Nemens E, White JA, et al.. Linkage and mutational analysis of familial Alzheimer disease kindreds for the APP gene region. Am J Hum Genet. 1992 Nov;51(5):998-1014. PMID:1415269
- ↑ Hendriks L, van Duijn CM, Cras P, Cruts M, Van Hul W, van Harskamp F, Warren A, McInnis MG, Antonarakis SE, Martin JJ, et al.. Presenile dementia and cerebral haemorrhage linked to a mutation at codon 692 of the beta-amyloid precursor protein gene. Nat Genet. 1992 Jun;1(3):218-21. PMID:1303239 doi:http://dx.doi.org/10.1038/ng0692-218
- ↑ Mullan M, Crawford F, Axelman K, Houlden H, Lilius L, Winblad B, Lannfelt L. A pathogenic mutation for probable Alzheimer's disease in the APP gene at the N-terminus of beta-amyloid. Nat Genet. 1992 Aug;1(5):345-7. PMID:1302033 doi:http://dx.doi.org/10.1038/ng0892-345
- ↑ Carter DA, Desmarais E, Bellis M, Campion D, Clerget-Darpoux F, Brice A, Agid Y, Jaillard-Serradt A, Mallet J. More missense in amyloid gene. Nat Genet. 1992 Dec;2(4):255-6. PMID:1303275 doi:http://dx.doi.org/10.1038/ng1292-255
- ↑ Liepnieks JJ, Ghetti B, Farlow M, Roses AD, Benson MD. Characterization of amyloid fibril beta-peptide in familial Alzheimer's disease with APP717 mutations. Biochem Biophys Res Commun. 1993 Dec 15;197(2):386-92. PMID:8267572
- ↑ Farlow M, Murrell J, Ghetti B, Unverzagt F, Zeldenrust S, Benson M. Clinical characteristics in a kindred with early-onset Alzheimer's disease and their linkage to a G-->T change at position 2149 of the amyloid precursor protein gene. Neurology. 1994 Jan;44(1):105-11. PMID:8290042
- ↑ Brooks WS, Martins RN, De Voecht J, Nicholson GA, Schofield PR, Kwok JB, Fisher C, Yeung LU, Van Broeckhoven C. A mutation in codon 717 of the amyloid precursor protein gene in an Australian family with Alzheimer's disease. Neurosci Lett. 1995 Oct 27;199(3):183-6. PMID:8577393
- ↑ Eckman CB, Mehta ND, Crook R, Perez-tur J, Prihar G, Pfeiffer E, Graff-Radford N, Hinder P, Yager D, Zenk B, Refolo LM, Prada CM, Younkin SG, Hutton M, Hardy J. A new pathogenic mutation in the APP gene (I716V) increases the relative proportion of A beta 42(43). Hum Mol Genet. 1997 Nov;6(12):2087-9. PMID:9328472
- ↑ Cras P, van Harskamp F, Hendriks L, Ceuterick C, van Duijn CM, Stefanko SZ, Hofman A, Kros JM, Van Broeckhoven C, Martin JJ. Presenile Alzheimer dementia characterized by amyloid angiopathy and large amyloid core type senile plaques in the APP 692Ala-->Gly mutation. Acta Neuropathol. 1998 Sep;96(3):253-60. PMID:9754958
- ↑ Ancolio K, Dumanchin C, Barelli H, Warter JM, Brice A, Campion D, Frebourg T, Checler F. Unusual phenotypic alteration of beta amyloid precursor protein (betaAPP) maturation by a new Val-715 --> Met betaAPP-770 mutation responsible for probable early-onset Alzheimer's disease. Proc Natl Acad Sci U S A. 1999 Mar 30;96(7):4119-24. PMID:10097173
- ↑ Finckh U, Muller-Thomsen T, Mann U, Eggers C, Marksteiner J, Meins W, Binetti G, Alberici A, Hock C, Nitsch RM, Gal A. High prevalence of pathogenic mutations in patients with early-onset dementia detected by sequence analyses of four different genes. Am J Hum Genet. 2000 Jan;66(1):110-7. PMID:10631141 doi:S0002-9297(07)62237-X
- ↑ Kwok JB, Li QX, Hallupp M, Whyte S, Ames D, Beyreuther K, Masters CL, Schofield PR. Novel Leu723Pro amyloid precursor protein mutation increases amyloid beta42(43) peptide levels and induces apoptosis. Ann Neurol. 2000 Feb;47(2):249-53. PMID:10665499
- ↑ Murrell JR, Hake AM, Quaid KA, Farlow MR, Ghetti B. Early-onset Alzheimer disease caused by a new mutation (V717L) in the amyloid precursor protein gene. Arch Neurol. 2000 Jun;57(6):885-7. PMID:10867787
- ↑ Kumar-Singh S, De Jonghe C, Cruts M, Kleinert R, Wang R, Mercken M, De Strooper B, Vanderstichele H, Lofgren A, Vanderhoeven I, Backhovens H, Vanmechelen E, Kroisel PM, Van Broeckhoven C. Nonfibrillar diffuse amyloid deposition due to a gamma(42)-secretase site mutation points to an essential role for N-truncated A beta(42) in Alzheimer's disease. Hum Mol Genet. 2000 Nov 1;9(18):2589-98. PMID:11063718
- ↑ Walsh DM, Hartley DM, Condron MM, Selkoe DJ, Teplow DB. In vitro studies of amyloid beta-protein fibril assembly and toxicity provide clues to the aetiology of Flemish variant (Ala692-->Gly) Alzheimer's disease. Biochem J. 2001 May 1;355(Pt 3):869-77. PMID:11311152
- ↑ Nilsberth C, Westlind-Danielsson A, Eckman CB, Condron MM, Axelman K, Forsell C, Stenh C, Luthman J, Teplow DB, Younkin SG, Naslund J, Lannfelt L. The 'Arctic' APP mutation (E693G) causes Alzheimer's disease by enhanced Abeta protofibril formation. Nat Neurosci. 2001 Sep;4(9):887-93. PMID:11528419 doi:10.1038/nn0901-887
- ↑ Pasalar P, Najmabadi H, Noorian AR, Moghimi B, Jannati A, Soltanzadeh A, Krefft T, Crook R, Hardy J. An Iranian family with Alzheimer's disease caused by a novel APP mutation (Thr714Ala). Neurology. 2002 May 28;58(10):1574-5. PMID:12034808
- ↑ Rossi G, Giaccone G, Maletta R, Morbin M, Capobianco R, Mangieri M, Giovagnoli AR, Bizzi A, Tomaino C, Perri M, Di Natale M, Tagliavini F, Bugiani O, Bruni AC. A family with Alzheimer disease and strokes associated with A713T mutation of the APP gene. Neurology. 2004 Sep 14;63(5):910-2. PMID:15365148
- ↑ Edwards-Lee T, Ringman JM, Chung J, Werner J, Morgan A, St George Hyslop P, Thompson P, Dutton R, Mlikotic A, Rogaeva E, Hardy J. An African American family with early-onset Alzheimer disease and an APP (T714I) mutation. Neurology. 2005 Jan 25;64(2):377-9. PMID:15668448 doi:64/2/377
- ↑ Miravalle L, Tokuda T, Chiarle R, Giaccone G, Bugiani O, Tagliavini F, Frangione B, Ghiso J. Substitutions at codon 22 of Alzheimer's abeta peptide induce diverse conformational changes and apoptotic effects in human cerebral endothelial cells. J Biol Chem. 2000 Sep 1;275(35):27110-6. PMID:10821838 doi:10.1074/jbc.M003154200
- ↑ Levy E, Carman MD, Fernandez-Madrid IJ, Power MD, Lieberburg I, van Duinen SG, Bots GT, Luyendijk W, Frangione B. Mutation of the Alzheimer's disease amyloid gene in hereditary cerebral hemorrhage, Dutch type. Science. 1990 Jun 1;248(4959):1124-6. PMID:2111584
- ↑ Grabowski TJ, Cho HS, Vonsattel JP, Rebeck GW, Greenberg SM. Novel amyloid precursor protein mutation in an Iowa family with dementia and severe cerebral amyloid angiopathy. Ann Neurol. 2001 Jun;49(6):697-705. PMID:11409420
- ↑ Greenberg SM, Shin Y, Grabowski TJ, Cooper GE, Rebeck GW, Iglesias S, Chapon F, Tournier-Lasserve E, Baron JC. Hemorrhagic stroke associated with the Iowa amyloid precursor protein mutation. Neurology. 2003 Mar 25;60(6):1020-2. PMID:12654973
- ↑ Obici L, Demarchi A, de Rosa G, Bellotti V, Marciano S, Donadei S, Arbustini E, Palladini G, Diegoli M, Genovese E, Ferrari G, Coverlizza S, Merlini G. A novel AbetaPP mutation exclusively associated with cerebral amyloid angiopathy. Ann Neurol. 2005 Oct;58(4):639-44. PMID:16178030 doi:10.1002/ana.20571
- ↑ Walter MF, Mason PE, Mason RP. Alzheimer's disease amyloid beta peptide 25-35 inhibits lipid peroxidation as a result of its membrane interactions. Biochem Biophys Res Commun. 1997 Apr 28;233(3):760-4. PMID:9168929 doi:10.1006/bbrc.1997.6547
- ↑ Kimberly WT, Zheng JB, Guenette SY, Selkoe DJ. The intracellular domain of the beta-amyloid precursor protein is stabilized by Fe65 and translocates to the nucleus in a notch-like manner. J Biol Chem. 2001 Oct 26;276(43):40288-92. Epub 2001 Sep 5. PMID:11544248 doi:10.1074/jbc.C100447200
- ↑ Rank KB, Pauley AM, Bhattacharya K, Wang Z, Evans DB, Fleck TJ, Johnston JA, Sharma SK. Direct interaction of soluble human recombinant tau protein with Abeta 1-42 results in tau aggregation and hyperphosphorylation by tau protein kinase II. FEBS Lett. 2002 Mar 13;514(2-3):263-8. PMID:11943163
- ↑ Nikolaev A, McLaughlin T, O'Leary DD, Tessier-Lavigne M. APP binds DR6 to trigger axon pruning and neuron death via distinct caspases. Nature. 2009 Feb 19;457(7232):981-9. PMID:19225519 doi:10.1038/nature07767
- ↑ Takuma K, Fang F, Zhang W, Yan S, Fukuzaki E, Du H, Sosunov A, McKhann G, Funatsu Y, Nakamichi N, Nagai T, Mizoguchi H, Ibi D, Hori O, Ogawa S, Stern DM, Yamada K, Yan SS. RAGE-mediated signaling contributes to intraneuronal transport of amyloid-beta and neuronal dysfunction. Proc Natl Acad Sci U S A. 2009 Nov 24;106(47):20021-6. doi:, 10.1073/pnas.0905686106. Epub 2009 Nov 9. PMID:19901339 doi:10.1073/pnas.0905686106
- ↑ Walter MF, Mason PE, Mason RP. Alzheimer's disease amyloid beta peptide 25-35 inhibits lipid peroxidation as a result of its membrane interactions. Biochem Biophys Res Commun. 1997 Apr 28;233(3):760-4. PMID:9168929 doi:10.1006/bbrc.1997.6547
- ↑ Kimberly WT, Zheng JB, Guenette SY, Selkoe DJ. The intracellular domain of the beta-amyloid precursor protein is stabilized by Fe65 and translocates to the nucleus in a notch-like manner. J Biol Chem. 2001 Oct 26;276(43):40288-92. Epub 2001 Sep 5. PMID:11544248 doi:10.1074/jbc.C100447200
- ↑ Rank KB, Pauley AM, Bhattacharya K, Wang Z, Evans DB, Fleck TJ, Johnston JA, Sharma SK. Direct interaction of soluble human recombinant tau protein with Abeta 1-42 results in tau aggregation and hyperphosphorylation by tau protein kinase II. FEBS Lett. 2002 Mar 13;514(2-3):263-8. PMID:11943163
- ↑ Nikolaev A, McLaughlin T, O'Leary DD, Tessier-Lavigne M. APP binds DR6 to trigger axon pruning and neuron death via distinct caspases. Nature. 2009 Feb 19;457(7232):981-9. PMID:19225519 doi:10.1038/nature07767
- ↑ Takuma K, Fang F, Zhang W, Yan S, Fukuzaki E, Du H, Sosunov A, McKhann G, Funatsu Y, Nakamichi N, Nagai T, Mizoguchi H, Ibi D, Hori O, Ogawa S, Stern DM, Yamada K, Yan SS. RAGE-mediated signaling contributes to intraneuronal transport of amyloid-beta and neuronal dysfunction. Proc Natl Acad Sci U S A. 2009 Nov 24;106(47):20021-6. doi:, 10.1073/pnas.0905686106. Epub 2009 Nov 9. PMID:19901339 doi:10.1073/pnas.0905686106
- ↑ Walter MF, Mason PE, Mason RP. Alzheimer's disease amyloid beta peptide 25-35 inhibits lipid peroxidation as a result of its membrane interactions. Biochem Biophys Res Commun. 1997 Apr 28;233(3):760-4. PMID:9168929 doi:10.1006/bbrc.1997.6547
- ↑ Kimberly WT, Zheng JB, Guenette SY, Selkoe DJ. The intracellular domain of the beta-amyloid precursor protein is stabilized by Fe65 and translocates to the nucleus in a notch-like manner. J Biol Chem. 2001 Oct 26;276(43):40288-92. Epub 2001 Sep 5. PMID:11544248 doi:10.1074/jbc.C100447200
- ↑ Rank KB, Pauley AM, Bhattacharya K, Wang Z, Evans DB, Fleck TJ, Johnston JA, Sharma SK. Direct interaction of soluble human recombinant tau protein with Abeta 1-42 results in tau aggregation and hyperphosphorylation by tau protein kinase II. FEBS Lett. 2002 Mar 13;514(2-3):263-8. PMID:11943163
- ↑ Nikolaev A, McLaughlin T, O'Leary DD, Tessier-Lavigne M. APP binds DR6 to trigger axon pruning and neuron death via distinct caspases. Nature. 2009 Feb 19;457(7232):981-9. PMID:19225519 doi:10.1038/nature07767
- ↑ Takuma K, Fang F, Zhang W, Yan S, Fukuzaki E, Du H, Sosunov A, McKhann G, Funatsu Y, Nakamichi N, Nagai T, Mizoguchi H, Ibi D, Hori O, Ogawa S, Stern DM, Yamada K, Yan SS. RAGE-mediated signaling contributes to intraneuronal transport of amyloid-beta and neuronal dysfunction. Proc Natl Acad Sci U S A. 2009 Nov 24;106(47):20021-6. doi:, 10.1073/pnas.0905686106. Epub 2009 Nov 9. PMID:19901339 doi:10.1073/pnas.0905686106
- ↑ Walter MF, Mason PE, Mason RP. Alzheimer's disease amyloid beta peptide 25-35 inhibits lipid peroxidation as a result of its membrane interactions. Biochem Biophys Res Commun. 1997 Apr 28;233(3):760-4. PMID:9168929 doi:10.1006/bbrc.1997.6547
- ↑ Kimberly WT, Zheng JB, Guenette SY, Selkoe DJ. The intracellular domain of the beta-amyloid precursor protein is stabilized by Fe65 and translocates to the nucleus in a notch-like manner. J Biol Chem. 2001 Oct 26;276(43):40288-92. Epub 2001 Sep 5. PMID:11544248 doi:10.1074/jbc.C100447200
- ↑ Rank KB, Pauley AM, Bhattacharya K, Wang Z, Evans DB, Fleck TJ, Johnston JA, Sharma SK. Direct interaction of soluble human recombinant tau protein with Abeta 1-42 results in tau aggregation and hyperphosphorylation by tau protein kinase II. FEBS Lett. 2002 Mar 13;514(2-3):263-8. PMID:11943163
- ↑ Nikolaev A, McLaughlin T, O'Leary DD, Tessier-Lavigne M. APP binds DR6 to trigger axon pruning and neuron death via distinct caspases. Nature. 2009 Feb 19;457(7232):981-9. PMID:19225519 doi:10.1038/nature07767
- ↑ Takuma K, Fang F, Zhang W, Yan S, Fukuzaki E, Du H, Sosunov A, McKhann G, Funatsu Y, Nakamichi N, Nagai T, Mizoguchi H, Ibi D, Hori O, Ogawa S, Stern DM, Yamada K, Yan SS. RAGE-mediated signaling contributes to intraneuronal transport of amyloid-beta and neuronal dysfunction. Proc Natl Acad Sci U S A. 2009 Nov 24;106(47):20021-6. doi:, 10.1073/pnas.0905686106. Epub 2009 Nov 9. PMID:19901339 doi:10.1073/pnas.0905686106
- ↑ Walter MF, Mason PE, Mason RP. Alzheimer's disease amyloid beta peptide 25-35 inhibits lipid peroxidation as a result of its membrane interactions. Biochem Biophys Res Commun. 1997 Apr 28;233(3):760-4. PMID:9168929 doi:10.1006/bbrc.1997.6547
- ↑ Kimberly WT, Zheng JB, Guenette SY, Selkoe DJ. The intracellular domain of the beta-amyloid precursor protein is stabilized by Fe65 and translocates to the nucleus in a notch-like manner. J Biol Chem. 2001 Oct 26;276(43):40288-92. Epub 2001 Sep 5. PMID:11544248 doi:10.1074/jbc.C100447200
- ↑ Rank KB, Pauley AM, Bhattacharya K, Wang Z, Evans DB, Fleck TJ, Johnston JA, Sharma SK. Direct interaction of soluble human recombinant tau protein with Abeta 1-42 results in tau aggregation and hyperphosphorylation by tau protein kinase II. FEBS Lett. 2002 Mar 13;514(2-3):263-8. PMID:11943163
- ↑ Nikolaev A, McLaughlin T, O'Leary DD, Tessier-Lavigne M. APP binds DR6 to trigger axon pruning and neuron death via distinct caspases. Nature. 2009 Feb 19;457(7232):981-9. PMID:19225519 doi:10.1038/nature07767
- ↑ Takuma K, Fang F, Zhang W, Yan S, Fukuzaki E, Du H, Sosunov A, McKhann G, Funatsu Y, Nakamichi N, Nagai T, Mizoguchi H, Ibi D, Hori O, Ogawa S, Stern DM, Yamada K, Yan SS. RAGE-mediated signaling contributes to intraneuronal transport of amyloid-beta and neuronal dysfunction. Proc Natl Acad Sci U S A. 2009 Nov 24;106(47):20021-6. doi:, 10.1073/pnas.0905686106. Epub 2009 Nov 9. PMID:19901339 doi:10.1073/pnas.0905686106
- ↑ Crescenzi O, Tomaselli S, Guerrini R, Salvadori S, D'Ursi AM, Temussi PA, Picone D. Solution structure of the Alzheimer amyloid beta-peptide (1-42) in an apolar microenvironment. Similarity with a virus fusion domain. Eur J Biochem. 2002 Nov;269(22):5642-8. PMID:12423364
|