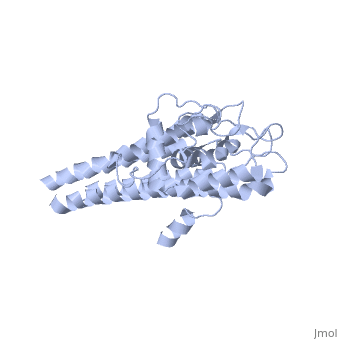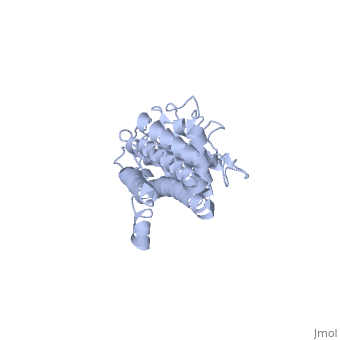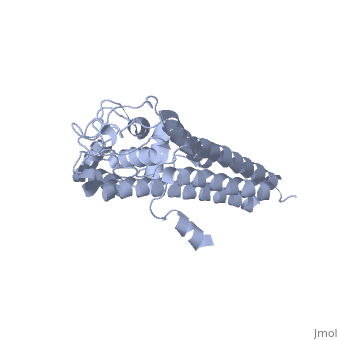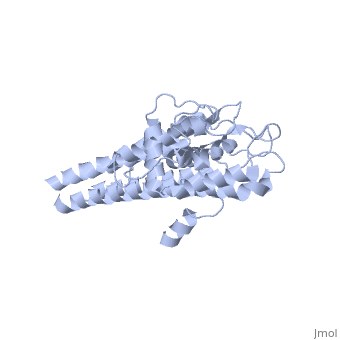
Structural highlights
Function
O06878_BORBG The Vlp and Vsp proteins are antigenically distinct proteins, only one vlp or vsp gene is transcriptionally active at any one time. Switching between these genes is a mechanism of host immune response evasion.[RuleBase:RU363105]
Evolutionary Conservation
Check, as determined by ConSurfDB. You may read the explanation of the method and the full data available from ConSurf.
Publication Abstract from PubMed
VlsE is an outer surface lipoprotein of Borrelia burgdorferi that undergoes antigenic variation through an elaborate gene conversion mechanism and is thought to play a major role in the immune response to the Lyme disease borellia. The crystal structure of recombinant variant protein VlsE1 at 2.3-A resolution reveals that the six variable regions form loop structures that constitute most of the membrane distal surface of VlsE, covering the predominantly alpha-helical, invariant regions of the protein. The surface localization of the variable amino acid segments appears to protect the conserved regions from interaction with antibodies and hence may contribute to immune evasion.
Crystal structure of Lyme disease variable surface antigen VlsE of Borrelia burgdorferi.,Eicken C, Sharma V, Klabunde T, Lawrenz MB, Hardham JM, Norris SJ, Sacchettini JC J Biol Chem. 2002 Jun 14;277(24):21691-6. Epub 2002 Mar 28. PMID:11923306[1]
From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.
References
- ↑ Eicken C, Sharma V, Klabunde T, Lawrenz MB, Hardham JM, Norris SJ, Sacchettini JC. Crystal structure of Lyme disease variable surface antigen VlsE of Borrelia burgdorferi. J Biol Chem. 2002 Jun 14;277(24):21691-6. Epub 2002 Mar 28. PMID:11923306 doi:10.1074/jbc.M201547200