Structural highlights
Disease
TRFE_HUMAN Defects in TF are the cause of atransferrinemia (ATRAF) [MIM:209300. Atransferrinemia is rare autosomal recessive disorder characterized by iron overload and hypochromic anemia.[1] [2]
Function
TRFE_HUMAN Transferrins are iron binding transport proteins which can bind two Fe(3+) ions in association with the binding of an anion, usually bicarbonate. It is responsible for the transport of iron from sites of absorption and heme degradation to those of storage and utilization. Serum transferrin may also have a further role in stimulating cell proliferation.
Publication Abstract from PubMed
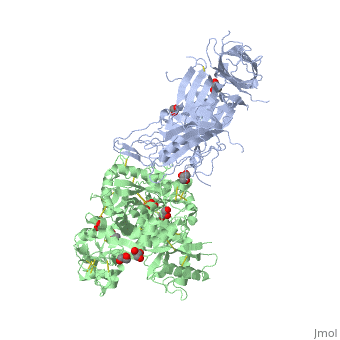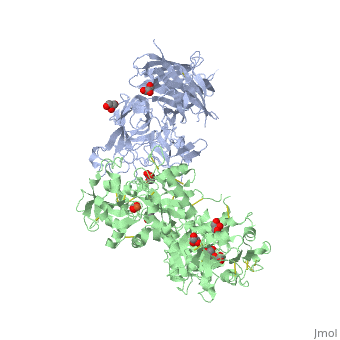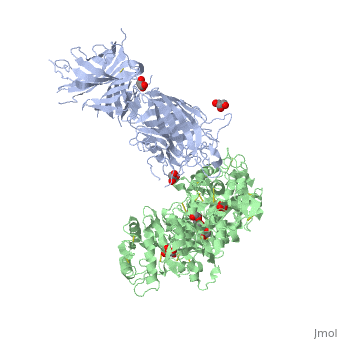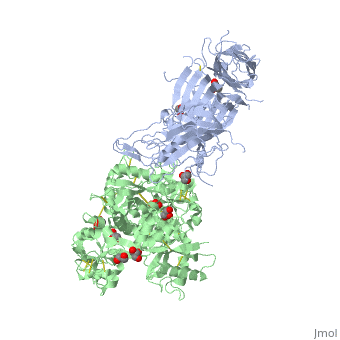
Neisseria meningitidis, the causative agent of bacterial meningitis, acquires the essential element iron from the host glycoprotein transferrin during infection through a surface transferrin receptor system composed of proteins TbpA and TbpB. Here we present the crystal structures of TbpB from N. meningitidis in its apo form and in complex with human transferrin. The structure reveals how TbpB sequesters and initiates iron release from human transferrin.
The structural basis of transferrin sequestration by transferrin-binding protein B.,Calmettes C, Alcantara J, Yu RH, Schryvers AB, Moraes TF Nat Struct Mol Biol. 2012 Feb 19;19(3):358-60. doi: 10.1038/nsmb.2251. PMID:22343719[3]
From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.
See Also
References
- ↑ Beutler E, Gelbart T, Lee P, Trevino R, Fernandez MA, Fairbanks VF. Molecular characterization of a case of atransferrinemia. Blood. 2000 Dec 15;96(13):4071-4. PMID:11110675
- ↑ Knisely AS, Gelbart T, Beutler E. Molecular characterization of a third case of human atransferrinemia. Blood. 2004 Oct 15;104(8):2607. PMID:15466165 doi:10.1182/blood-2004-05-1751
- ↑ Calmettes C, Alcantara J, Yu RH, Schryvers AB, Moraes TF. The structural basis of transferrin sequestration by transferrin-binding protein B. Nat Struct Mol Biol. 2012 Feb 19;19(3):358-60. doi: 10.1038/nsmb.2251. PMID:22343719 doi:10.1038/nsmb.2251