Structural highlights
Function
FUMC_MYCTU Catalyzes the reversible addition of water to fumarate to give L-malate.
Publication Abstract from PubMed
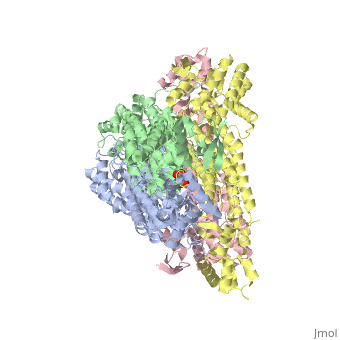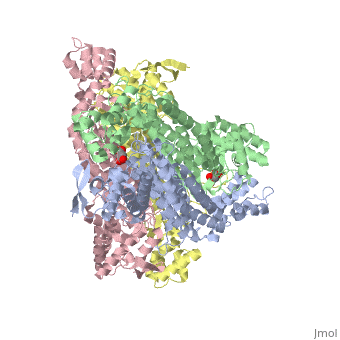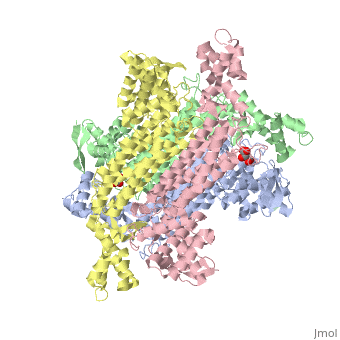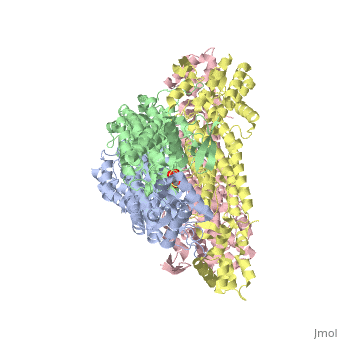
rv1098c, an essential gene in Mycobacterium tuberculosis, codes for a class II fumarase. We describe here the crystal structure of Rv1098c in complex with l-malate, fumarate or the competitive inhibitor meso-tartrate. The models reveal that substrate binding promotes the closure of the active site through conformational changes involving the catalytic SS-loop and the C-terminal domain, which likely represents a general feature of this enzyme superfamily. Analysis of ligand-enzyme interactions as well as site-directed mutagenesis suggest Ser318 as one of the two acid-base catalysts. STRUCTURED SUMMARY OF PROTEIN INTERACTIONS: Rv1098c and Rv1098c bind by X-ray crystallography (View interaction).
Conformational changes upon ligand binding in the essential class II fumarase Rv1098c from Mycobacterium tuberculosis.,Mechaly AE, Haouz A, Miras I, Barilone N, Weber P, Shepard W, Alzari PM, Bellinzoni M FEBS Lett. 2012 Jun 4;586(11):1606-11. Epub 2012 May 3. PMID:22561013[1]
From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.
See Also
References
- ↑ Mechaly AE, Haouz A, Miras I, Barilone N, Weber P, Shepard W, Alzari PM, Bellinzoni M. Conformational changes upon ligand binding in the essential class II fumarase Rv1098c from Mycobacterium tuberculosis. FEBS Lett. 2012 Jun 4;586(11):1606-11. Epub 2012 May 3. PMID:22561013 doi:10.1016/j.febslet.2012.04.034