4f38
From Proteopedia
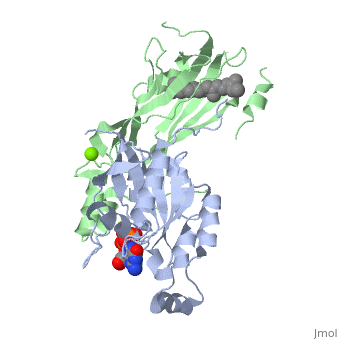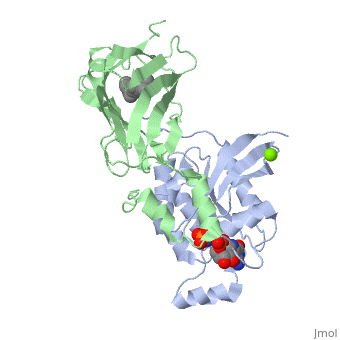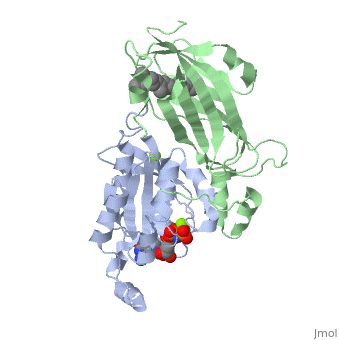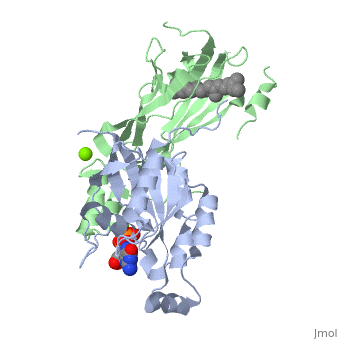
Crystal structure of geranylgeranylated RhoA in complex with RhoGDI in its active GPPNHP-bound form
Structural highlights
FunctionRHOA_MOUSE Small GTPase which cycles between an active GTP-bound and an inactive GDP-bound state (PubMed:14697203). Mainly associated with cytoskeleton organization, in active state binds to a variety of effector proteins to regulate cellular responses such as cytoskeletal dynamics, cell migration and cell cycle. Regulates a signal transduction pathway linking plasma membrane receptors to the assembly of focal adhesions and actin stress fibers. Involved in a microtubule-dependent signal that is required for the myosin contractile ring formation during cell cycle cytokinesis (By similarity). Plays an essential role in cleavage furrow formation. Required for the apical junction formation of keratinocyte cell-cell adhesion (PubMed:11777936, PubMed:20974804). Essential for the SPATA13-mediated regulation of cell migration and adhesion assembly and disassembly. The MEMO1-RHOA-DIAPH1 signaling pathway plays an important role in ERBB2-dependent stabilization of microtubules at the cell cortex. It controls the localization of APC and CLASP2 to the cell membrane, via the regulation of GSK3B activity. In turn, membrane-bound APC allows the localization of the MACF1 to the cell membrane, which is required for microtubule capture and stabilization (By similarity). Regulates KCNA2 potassium channel activity by reducing its location at the cell surface in response to CHRM1 activation; promotes KCNA2 endocytosis. Acts as an allosteric activator of guanine nucleotide exchange factor ECT2 by binding in its activated GTP-bound form to the PH domain of ECT2 which stimulates the release of PH inhibition and promotes the binding of substrate RHOA to the ECT2 catalytic center (By similarity). May be an activator of PLCE1 (PubMed:9635436). In neurons, involved in the inhibition of the initial spine growth. Upon activation by CaMKII, modulates dendritic spine structural plasticity by relaying CaMKII transient activation to synapse-specific, long-term signaling (By similarity). Acts as a regulator of platelet alpha-granule release during activation and aggregation of platelets (PubMed:14697203). When activated by DAAM1 may signal centrosome maturation and chromosomal segregation during cell division. May also be involved in contractile ring formation during cytokinesis.[UniProtKB:P61586][UniProtKB:P61589][1] [2] [3] [4] [5] Publication Abstract from PubMedSmall GTPases of the Rho family regulate cytoskeleton remodeling, cell polarity, and transcription, as well as the cell cycle, in eukaryotic cells. Membrane delivery and recycling of the Rho GTPases is mediated by Rho GDP dissociation inhibitor (RhoGDI), which forms a stable complex with prenylated Rho GTPases. We analyzed the interaction of RhoGDI with the active and inactive forms of prenylated and unprenylated RhoA. We demonstrate that RhoGDI binds the prenylated form of RhoA.GDP with unexpectedly high affinity (K(d) = 5 pm). The very long half-life of the complex is reduced 25-fold on RhoA activation, with a concomitant reduction in affinity (K(d) = 3 nm). The 2.8-A structure of the RhoA.guanosine 5'-[beta,gamma-imido] triphosphate (GMPPNP).RhoGDI complex demonstrated that complex formation forces the activated RhoA into a GDP-bound conformation in the absence of nucleotide hydrolysis. We demonstrate that membrane extraction of Rho GTPase by RhoGDI is a thermodynamically favored passive process that operates through a series of progressively tighter intermediates, much like the one that is mediated by RabGDI. Quantitative Analysis of Prenylated RhoA Interaction with Its Chaperone, RhoGDI.,Tnimov Z, Guo Z, Gambin Y, Nguyen UT, Wu YW, Abankwa D, Stigter A, Collins BM, Waldmann H, Goody RS, Alexandrov K J Biol Chem. 2012 Aug 3;287(32):26549-62. Epub 2012 May 24. PMID:22628549[6] From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine. See AlsoReferences
| ||||||||||||||||||||