Function
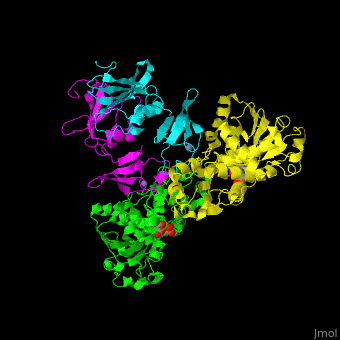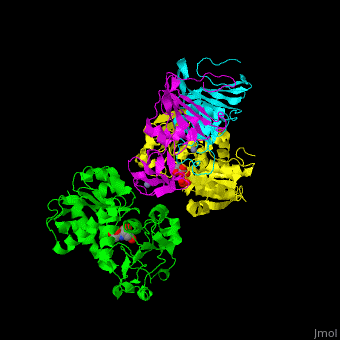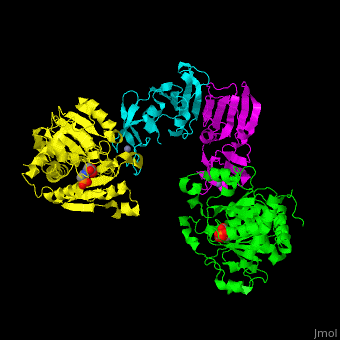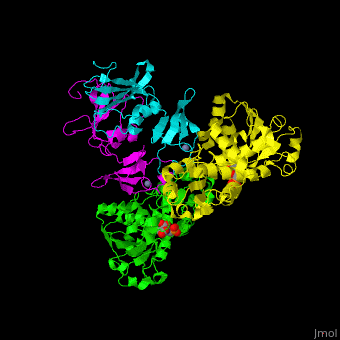
Aspartate carbamoyltransferase (ATC) is part of the pyrimidine biosynthesis pathway. ATC catalyzes the condensation of aspartate and carbamoyl phosphate to N-carbamyl-L-aspartate and phosphate. The Zn atom is essential for the association of the subunits. Binding of the substrate to the catalytic subunits results in a high-affinity state while binding of CTP to the regulatory subunit results in a low-affinity state. Malate and phosphonoacetyl-L-aspartate (PALA) are inhibitors of ATC. For additional details see Aspartate Transcarbamoylase (ATCase).
Structural highlights
ATC is composed of . Click to see and . The contains an and a carbamoyl-phosphate-binding domain. The regulatory subunit contains a nucleotide effectors-binding domain and a zinc domain. [1]
3D structures of aspartate carbamoyltransferase
Aspartate carbamoyltransferase 3D structures