BASIL2023GVP30646
From Proteopedia
Characterization of Novel Xylulokinase P30646
|
Contents |
Abstract
The Protein Data Bank (PDB) and UniProt contain a wealth of protein structures, many of which do not have a known function. One such protein is P30646, an uncharacterized sugar kinase. Sugar kinases play a role in a variety of metabolic processes within the body, so understanding their functions is of great importance. Our work aimed to further characterize P30646 through the identification of a substrate for this enzyme. Utilizing materials Biochemistry Authentic Scientific Inquiry Laboratory (BASIL) project, our group combined in silico and in vitro data for a full analysis of P30646. Computation tools including BLAST, DALI, SPRITE, and InterPro allowed our group to compare the structural and sequential similarities between P30646 and proteins with known functions. In silico analysis indicated that P30646 had xylulokinase activity, and xylulose was tested as a substrate in vitro using a coupled kinase assay. The kinase assay indicated that P30646 does have strong enzymatic activity for xylulose. The combination of our in silico and in vitro results provide a strong indication that P30646 is a sugar kinase with affinity for xylulose breakdown.
Background
The study of protein structure and function is essential, as the structure of a protein is often closely related to its function. This project investigated the protein P30646 (UniProt), a protein with a known structure but an unknown function. It is understood that P30646 is an uncharacterized sugar kinase that is involved in the metabolic processes of Caenorhabditis elegans. Through the analysis of results obtained through computational and laboratory methods, this work aims to identify the function of P30646 and gain insight into its role in the metabolism of C. elegans.
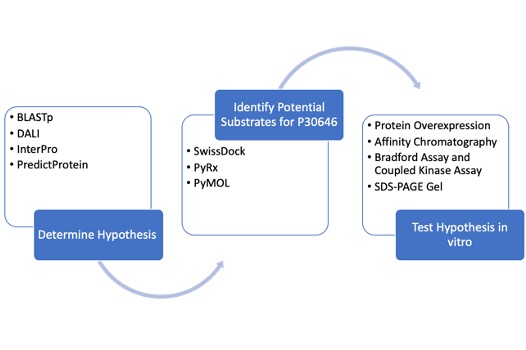
Workflow
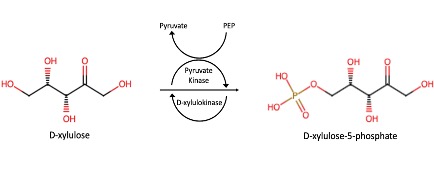
Predicted Mechanism
Methods
To determine the function of P30646 as well as a potential substrate for the enzyme, we first conducted an in silico exploration. We utilized tools including DALI, InterPro, PredictProtein, and BLASTp to gain insight into the potential function P30646. Each of these tools draws upon data for proteins of unknown functions to generate hypotheses about the function of the protein of interest. We then identified potential substrates for our protein of interest through docking tools including SwissDock and PyRx.
The hypotheses we developed through computational tools were tested in vitro by overexpressing and purifying P30646, followed by an experimental analysis of the protein. This analysis involved a Bradford assay, a coupled kinase assay, and SDS-PAGE.
Computational Exploration
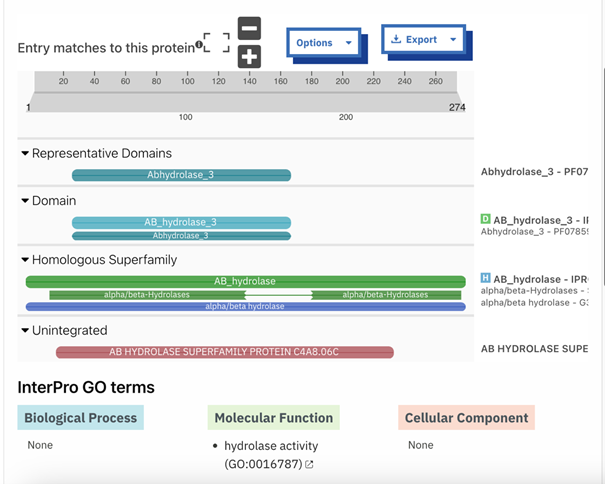
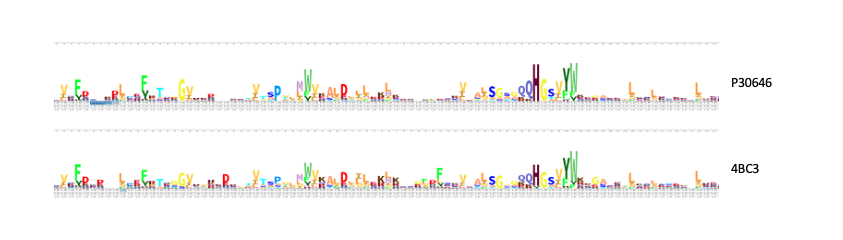
Our in silico experiments focused on first determining a potential function for our protein of interest, and then on identifying an ideal substrate. We hypothesized about a function for P30646 through structural and sequential alignments with proteins of known function. A number of similar proteins were identified, including a 4BC3 (PDB), a human D-xylulokinase. 4BC3, along with other xylulokinases, is both structurally and sequentially similar to P30646. Results for the protein family membership of P30646, as well as a sequential alignment with 4BC3 are provided below.
A combination of all in silico data collected led us to conclude that our protein of interest is a D-xylulokinase.
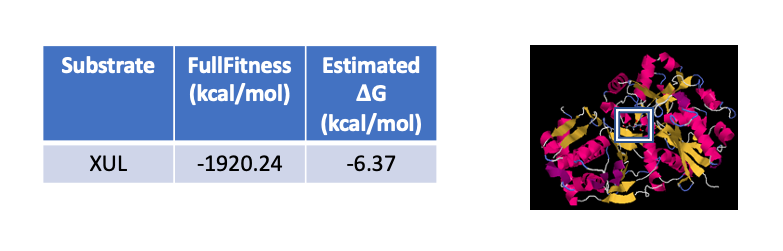
Our hypothesis that P30646 is a D-xylulokinase implies that D-xylulose would be an ideal substrate for the protein. In order to test this, our group utilized SwissDock and PyRx to conduct in silico docking experiments. Other substrates tested included ethylene glycol, 5-deoxy-5-fluoro-D-xylulose, and ammonium and sulfate ions. Docking of D-xylulose to P30646 is shown below.
Both tools supported our prediction that D-xylulose was an ideal substrate for P30646, and we moved forward to test our hypotheses in the laboratory.
Laboratory Experiments
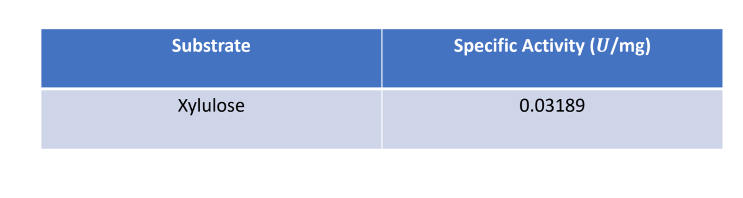
In order to test our hypotheses in vitro, we first performed an overexpression of our protein of interest followed by purification using affinity chromatography. Our group then tested D-xylulose for its fit as a substrate for P30646. The results of a coupled kinase assay for P30646 with D-xylulose as a potential substrate are shown below.
The results of our coupled kinase assay showed us that P30646 aids in the phosphorylation of D-xylulose, as demonstrated in the predicted mechanism for our protein of interest. While it is clear that D-xylulose is a potential substrate for P30646, it is unclear whether or not this is the best substrate for P30646 without further testing.
Conclusions
Through a combination of the data collected in vitro and in silico, our group concluded that P30646 is a D-xylulokinase. Computational tools aided in this determination by indicating that P30646 belongs to the carbohydrate kinase family, and more specifically to the D-xylulokinase family. Alignment with similar D-xylulokinases further supported this idea.
Evidence from the coupled kinase assay led our group to determine that D-xylulose is a possible substrate for P30646. The data collected indicates that P30646 has the capability to phosphorylate D-xylulose, though the specific activity of the enzyme is lower than expected. Increased testing using a variety of carbohydrates is necessary to make further conclusions regarding D-xylulose as a substrate for P30646.
References
1. BASIL. https://basilbiochem.github.io/basil/
2. Blastp [Internet]. Bethesda (MD): Natiobal Library of Medicine (US), National Center for Biotechnology Information; 2004- [cited 2022 March]. Available from: (https://blast.ncbi.nlm.nih.gov/Blast.cgi?PAGE=Proteins)
3. Di Luccio, Eric et al. Journal of Molecular Biology vol. 365,3 (2007): 783-98. doi:10.1016/j.jmb.2006.10.068
4. The PyMOL Molecular Graphics System, Version 1.2r3pre, Schrödinger, LLC.
5. The use of JSmol in Proteopedia [1].
Proteopedia Page Contributors and Editors (what is this?)
Isabelle Juhler, Asal Eid, Bonnie Hall, Jaime Prilusky
DOI: https://dx.doi.org/10.14576/957645.4038173 (?)Citation: Juhler I, Eid A, Hall B, 2024, "BASIL2023GVP30646",