From Proteopedia
proteopedia linkproteopedia link The Effect of Caffeine (Trimethylxanthine) on Human A2A Receptor
| Caffeine is a stimulant that helps temporarily increase alertness as well as energy. It is found in several plants; most commonly in the plant leaves and seeds. It can also be artificially created and added. Within the human body, Caffeine can affect the CNS for up to 6 hours. It binds to Adenosine receptors and inhibits their effects allowing for more attentiveness (Xu and Stevens, 2011).
Caffeine (Trimethylxanthine)
Caffeine, systematic name is 1,3,7-trimethylxanthine, is a xanthine derivative. It is composed of purines; structurally it is polar, and water soluble (Figure 1). They antagonize or inhibit many of the adenosine receptors, like the A2A receptor. Caffeine affects neurons and glial cells in the brain by binding to the same location that adenosine would bind and induce a cascade of enzymatic downstream effects (Denoeud et al 2014).
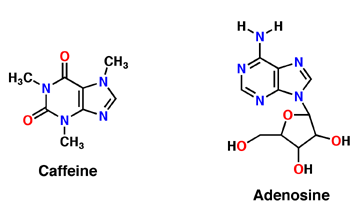
Figure 1: The molecular structure of Caffeine and Adenosine.
Mechanism of Caffeine (Trimethylxanthine) Synthesis
Caffeine is a naturally occurring methylxanthine, purine alkaloid, synthesized by eudicot plants such as coffee, cacao, and tea (Denoeud et. al, 2014). In order to synthesize caffeine, xanthosine must undergo 3 methylation steps with the help of three NMT enzymes (Figure 2); xanthosine methyltransferase (XMT), theobromine synthase (MXMT), and caffeine synthase (DXMT) (Denoeud et. al, 2014). The first step of caffeine biosynthesis involves XMT converting S-adenosylmethionine (SAM) to S-adenosylhomocysteine (SAH) which removes a methyl group and adds it to the 7’-Nitrogen. This produces the intermediate 7-methyl-xanthosine to become 7-methyl-xanthine (Denoeud et al 2014). The second enzyme, MXMT, converts another SAM to SAH, subsequently adding a methyl group to the 3’- Nitrogen on 7-methyl-xanthine. This produces theobromine which undergoes another methylation step with the help of the enzyme DXMT. DXMT converts a third SAM to SAH, adding a methyl group to the 1’-Nitrogen, yielding a caffeine molecule (Denoeud et al 2014).
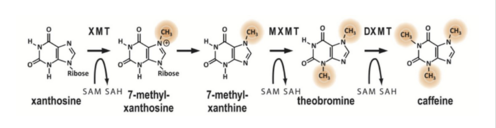
Figure 2. The synthesis of Caffeine from Xanthosine.
Adenosine
Adenosine is an inhibitory neurotransmitter, which promotes sleep and inhibits arousal. It has two components; an adenine nucleotide and a ribose sugar (Figure 1). Adenosine is a polar molecule and is water soluble. Within the brain, concentration of this neuromodulator increases every hour. Adenosine binds intracellularly to G-protein and induces multiple effects. As adenosine receptors bind G-protein, neural activity begins to decrease and the person feels fatigued and sleepy. A2A receptor is one of many adenosine G protein-coupled receptors (Huang et al 2014).
Four Different Adenosine Receptors
There are four different types of adenosine receptors that bind and activate adenosine, the ligand for that receptor. The four types, A1, A2B, A3 and A2A are all members of the G protein coupled receptor, which is a membrane spanning protein. These receptors are expressed in the brain, immune system, and cardiovascular system. The receptor, A1, protects the heart from an oxygen deficiency, slowing down the heart rate. When adenosine binds to the A1 receptor, it causes a cascade of effects, such as reduces the cyclic AMP level, increase calcium concentration, and increases ERK1 and ERK 2 (kinases that help with cell growth and differentiation) (Antonioli et al 2013).
The A2B receptor is an integral membrane protein that, when in the presence of adenosine, stimulates adenylate cyclase. A2B also is involved in axon elongation, by interacting with the protein netrin-1 (ADORA2B Adenosine A2B Receptor [ Homo Sapiens (human) ].) The A3 receptor, also a G- protein linked receptor, is involved in the cell growth and division, and cell death. This receptor also has both neurodegenerative and neuroprotective effects ("ADORA3 Adenosine A3 Receptor [ Homo Sapiens (human) ]).
The A2A receptor improves the flow of blood to the heart, increasing heart rate, and additionally can lower blood pressure. When adenosine binds to the A2A receptor, cyclicAMP levels increase, and ERK1/ERK2 levels increase (Antonioli et al 2013).
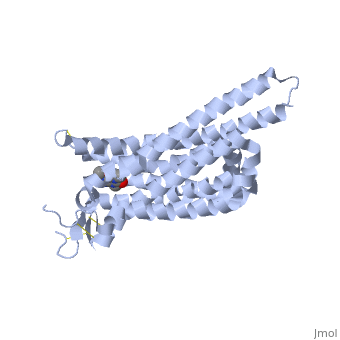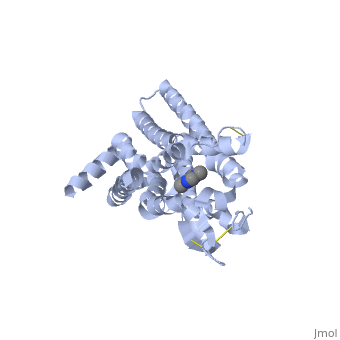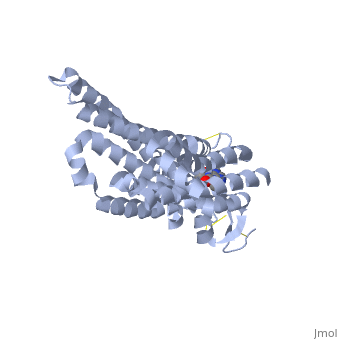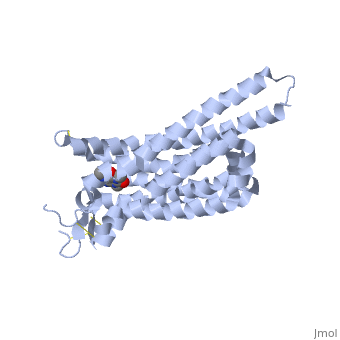
Structure of Adenosine (A2A) Receptor
The adenosine receptor (A2A) is a G-protein coupled receptor, which is a transmembrane protein that consists of secondary structures, such as seven alpha helical domains. Inside the third and seventh transmembrane helical domains, there are hydrophobic side chains that are required for ligand recognition. The target ligand, adenosine, is a large, polar molecule that binds to the extracellular binding domain of the A2A receptor by several nonpolar interactions. To be specific, these nonpolar interactions include hydrogen bonding (11), aromatic stacking interactions (1), and many van der Waals interactions (Xu et al 2011). To avoid the steric interactions between the ribose ring of adenosine and the tryptophan of the enzyme binding pocket, these nonpolar interactions cause conformational changes within the binding cavity, and cause an internal rotation and tilt of the seventh helical domain (Xu et al 2011). Other molecules, such as caffeine can also bind to these adenosine receptors. When caffeine binds to this receptor, it inhibits adenosine from binding to the extracellular binding domain of the A2A receptor.
How Caffeine (Trimethylxanthine) Binds
A2A is a transmembrane protein coupled with a G protein in humans. Trimethylxanthine is highly water soluble and thus when present in the system, interacts with the A2A receptor. In order for Trimethylxantine to bind to the receptor, the third and seventh transmembrane helical domains need to recognize the ligand. Trimethylxanthine can then bind. Trimethylxanthine, due to its similar structure and its purine alkaloid structure, can bind in place of adenosine. This binding changes the shape, but does not initiate the cascade of downstream effects that adenosine does, like opening of ion channels and slowing its activity. The concentrate of free adenosine increases extracellularly, when trimethylxanthine is bound (Xu and Stevens, 2011).
cAMP levels increase when adenosine is bound and are not effected when caffeine is bound. ERK1 and ERK2 are kinases, which modify serine and threonine, of the GMGC group that regulation of cell growth and differentiation, and if adenosine was bound, this cascade of events would occur, but when Trimethylxanthine is bound, this regulation does not occur (Xu and Stevens, 2011).
Conclusion
Caffeine increases temporary alertness, energy, and mood. Sensitivity to caffeine is different from person to person. It will be more effective on a smaller individual than a larger individual. While considered safe in small quantities, caffeine can cause irritability and headaches if someone consumes over 300 mg per day. This intake can be reduced by consumption of non-caffeinated coffee, water and tea (Dore et al 2011). Caffeine is still largely misunderstood, and in the next few years, more and more studies will be conducted due to caffeine becoming a larger factor in today’s school and work places (Olsen, 2013).
See Also
http://www.nature.com/nrc/journal/v13/n12/fig_tab/nrc3613_F1.html
http://www.rcsb.org/pdb/explore/explore.do?structureId=4UHR
http://www.rcsb.org/pdb/explore/explore.do?structureId=3RFM
http://www.edb.utexas.edu/ssn/SN%20PDF/Caffeine-Exercise%20Perform.PDF
http://www.ncbi.nlm.nih.gov/pubmed/20164566
http://www.pnas.org/content/112/25/7833.short
http://www.ncbi.nlm.nih.gov/pubmed/1356551
|
References
Antonioli, L., Blandizzi, C., Pacher, P. and Haskó, G. (2013). "Adensoine and Adenosine Receptors" Nature Publishing Group. Web.
"ADORA2B Adenosine A2b Receptor [ Homo Sapiens (human) ]." NCIB. N.p., n.d. Web. 16 Nov. 2015.
"ADORA3 Adenosine A3 Receptor [ Homo Sapiens (human) ]." NCBI. N.p., n.d. Web. 16 Nov. 2015.
Denoeud, F., Carretero-Paulet, A., Dereeper, G., Droc, R., Guyot, M., Pietrella, C., Zheng, A., Alberti, F., Anthony, G., Aprea, J.-M., Aury, P., Bento, M., Bernard, S., Bocs, C., Campa, A., Cenci, M.-C., Combes, D., Crouzillat, C., Da-Silva, L., Daddiego, F., De-Bellis, S., Dussert, O., Garsmeur, T., Gayraud, V., Guignon, K., Jahn, V., Jamilloux, T., Joet, K., Labadie, T., Lan, J., Leclercq, M., Lepelley, T., Leroy, L.T., Li, P., Librado, L., Lopez, A., Munoz, B., Noel, A., Pallavicini, G., Perrotta, V., Poncet, D., Pot, M., Priyono, M., Rigoreau, M., Rouard, J., Rozas, C., Tranchant-Dubreuil, R., Vanburen, Q., Zhang, A.C., Andrade, X., Argout, B., Bertrand, A., De Kochko, G., Graziosi, R. J., Henry, J., Jayarama, R., Ming, C., Nagai, S., Rounsley, D., Sankoff, G., Giuliano, V.A., Albert, P., Wincker, P. and Lashermes, P. (2014). "The Coffee Genome Provides Insight into the Convergent Evolution of Caffeine Biosynthesis" Science. 345.6201: 1181-184.
Doré, A.S. and Marshall, F.H. (2011). "Structure of the adenosine A2A receptor in complex with ZM241385 and the xanthines XAC and caffeine" Elsevier. 19.01: 1283–1293
Huang, Z.L., Zhang, Z. and Qu, W.M. (2014). "Roles of adenosine and its receptors in sleep-wake regulation" International Review Neurobiology. 119.001: 349-371.
Mitchell, E. (2014). "Caffeine: Convergently Evolved or Creatively Provided". Digital image. Web.
Oslen, N.L. (2013). "Caffeine Consumption Habits and Perceptions among University of New Hampshire Students" University of New Hampshire Scholars. 103.001: Print.
Xanthine. Digital image. LookForDiagnosis. N.p., Sept. 2014. Web. <http://www.lookfordiagnosis.com/mesh_info.php?term=Xanthine&lang=1>.
Xu, F. and Stevens, R.C. (1993). “Trapping Small Caffeine in a Large GPCR Pocket” Elsevier. 19.09: 1204–1207. Web.
Xu, F., Wu, H., Katritch, V., Han, G.W., Jacobson, K.A., Gao, Z.G., Cherezov, V. and Stevens, R.C. (2011). "Structure of an Agonist-Bound Human A2A Adenosine Receptor" (n.d.): n. pag. Web.