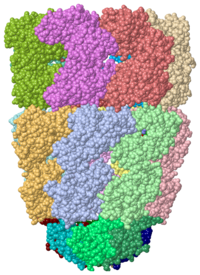
Crystal Structure of Chaperonin,
1pcqChaperonins (Cpn) are oligomeric proteins that mediate the folding of polypeptide chains. Group I CPN are found in bacteria, chloroplasts and mitochondria. For an introductory overview, see Chaperonins in Wikipedia.
The most characterized Cpn are in the GroEL/GroES complex from Escherichia coli and Cpn60/Cpn10 from Thermus thermophilus.[1] The larger subunit (GroEL, Cpn60) contains 3 domains: apical, intermediate and equatorial domain. The apical domain is the one which binds the polypeptide substrate. The equatorial domains binds the nucleotide. Group II Cpns are found in eukaryotic cytosol and archaea. Thermosome is a Cpn complex found in archaea.[2] CCT or TRiC is a Cpn complex found in eukarya.[3]
3D Structures of Chaperonin
Chaperonin 3D structures
Files for 3D printer
Asymmetric Chaperonin Complex GroEL/GroES by Marius Mihasan

