Function
DAHP synthase or 3-deoxy-D-arabino-heptulosonate 7-phosphate synthase or phospho-2-dehydro-3-deoxyheptonate aldolase (DAHPS) catalyzes the conversion of phosphoenolpyruvate (PEP) and D-erythrose 4-phosphate to 3-deoxy-D-arabino-heptulosonate 7-phosphate (DAHP) and phosphate. DAHPS is part of the shikimate pathway. DAHPS requires a bivalent metal ion cofactor for normal activity. . DAHPS exhibits feedback inhibition by aromatic amino acids like tyrosine, phenylalanine and tryptophan.[1]
Structural highlights
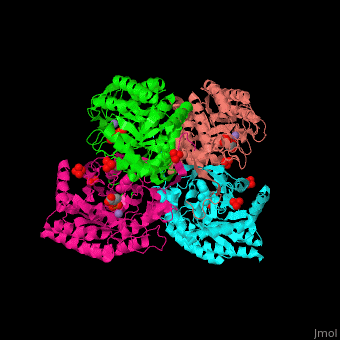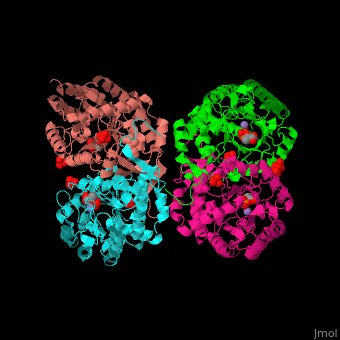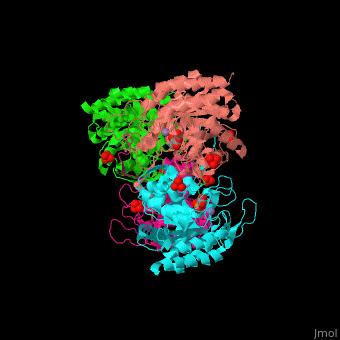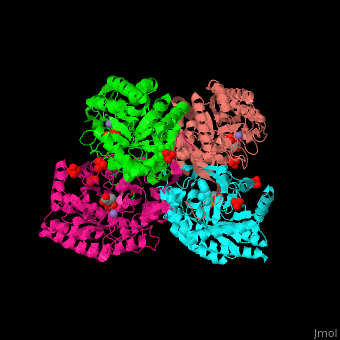
The DAHPS active site is located in a channel at the C-terminal of the enzyme where the , and are seen. The bivalent metal is bound to a Cys-X-X-His motif.[2]
3D Structures of DAHP synthase
DAHP synthase 3D structures