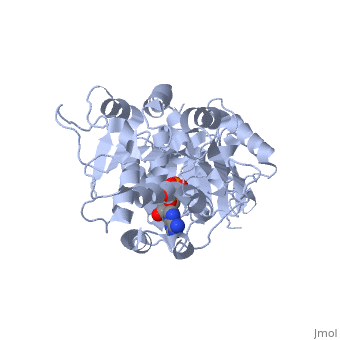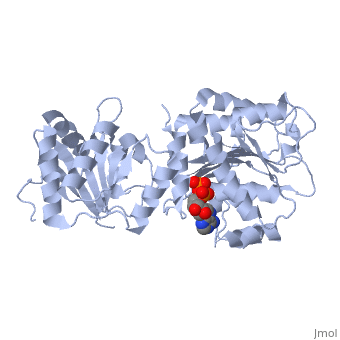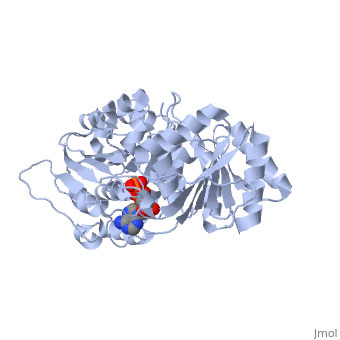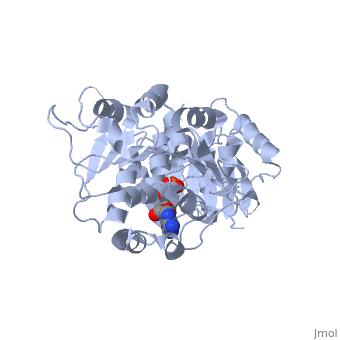
Human DEAD-box RNA-helicase DDX19 in complex with ADP (3ews)
Publication Abstract from PubMed
DEXD/H-box RNA helicases couple ATP hydrolysis to RNA remodeling by an unknown mechanism. We used x-ray crystallography and biochemical analysis of the human DEXD/H-box protein DDX19 to investigate its regulatory mechanism. The crystal structures of DDX19, in its RNA-bound prehydrolysis and free posthydrolysis state, reveal an alpha-helix that inserts between the conserved domains of the free protein to negatively regulate ATPase activity. This finding was corroborated by biochemical data that confirm an autoregulatory function of the N-terminal region of the protein. This is the first study describing crystal structures of a DEXD/H-box protein in its open and closed cleft conformations.
The DEXD/H-box RNA helicase DDX19 is regulated by an {alpha}-helical switch., Collins R, Karlberg T, Lehtio L, Schutz P, van den Berg S, Dahlgren LG, Hammarstrom M, Weigelt J, Schuler H, J Biol Chem. 2009 Apr 17;284(16):10296-300. Epub 2009 Feb 25. PMID:19244245
From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.
About this Structure
3EWS is a 2 chains structure of sequences from Homo sapiens. Full crystallographic information is available from OCA.
The is a single-chain DDX19 monomer. .
DDX19 is .
- With ADP bound, an N-terminal extension (dark green) is wedged in a cleft between these domains negatively regulating ATPase activity by preventing cleft closure.
- This conformation is presumed to resemble the post-hydrolysis state in which RNA is not bound. It is also referred to as the open state and open cleft conformation.
- This scene is similar to the view in Figure 1a of the paper describing the structure. ( because Arginine 429, discussed below, is included as in that figure.)
Showing
are bound.
- This overlay clearly illustrates the dramatic shift in the location of the N-terminal extension (dark green).
- This view clearly shows how the N-terminal helix of the extension extends from the RNA-binding site to down near the ATP binding site.
. The placement of the N-terminal extension between the two helicase domains negatively regulates ATPase activity. A .
- Of particular note is arginine 429, the so-called arginine finger, that is essential for ATPase activity. due to the helix of the extension sitting in the cleft.
- illustrates how it is moved to sit near the active site when RNA is bound, enabling ATPase activity.
- Keep in mind the intermediate models, in between the endpoints, are hypothetical.
See also
- Helicase
- 3g0h is human DDX19 with a non-hydrolyzable ATP analog and mRNA mimic bound. It is used in the morphs here and was described in the same article as 3ews.