Introduction
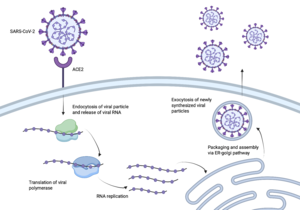
Figure 1: The process of the SARS-CoV-2 virus entering human cells. Image created using BioRender.
SARS-CoV-2, better known as Covid-19, sent the world into a global pandemic due to its rapid transmission and infection. In order to create a vaccine, it was essential to understand how SARS-CoV-2 infected us.
The enzyme angiotensin converting enzyme 2 is attached to our cell membranes and then can be bound to a receptor binding domain [1]. The virus, SARS-CoV-2, enters our bodies containing a protruding from the viral membrane. The RBD on the end of the spike protein , giving SARS-CoV-2 the ability to enter our host cells [2]. Once the spike protein has access to our host cells, it is able to further infect our cells and spread the virus throughout our bodies. Figure 1 demonstrates the path SARS-CoV-2 takes to get into our cells. In order to create an effective vaccine, the pathway between the spike protein and the RBD needed to be interrupted [3].
Vaccines
A vaccine activates the body’s immune system by containing weakened parts of viruses. This activation stimulates the production of antibodies. then compete with the ACE2 protein for the binding of viral spike proteins. In the case of SARS-CoV-2 spike proteins, once they enter the body they
to the Fab fragment of the antibody, a 90% neutralizing response for targeting the RBD is created. In this scene only the Fab fragment is being shown due to the fact that the Fab fragment is the part of the antibody that interacts with the RBD. With this being said, this method of treatment is difficult for long term use due to the evolution of the viral cells [4].
Protein Inhibitor Development
Protein inhibitors were thought of as a new idea for creating vaccines due to their smaller size and better stability compared to antibody vaccines[5]. These protein inhibitors, also referred to as mini-binders, interact with the receptor binding domain of the spike protein,
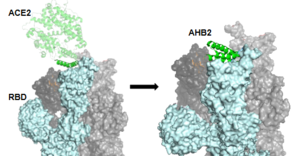
Figure 2: The use of the Rosetta Blueprint protein design to create the AHB2 inhibitor (7JZL & 7UHB).
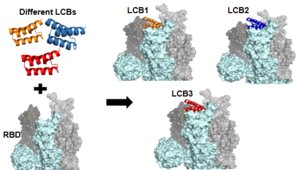
Figure 3:The use of the De Novo protein design to create the LCB1 and LCB3 inhibitors (7JZL).
The Process of Discovery
The first mini-binder to be created to combat COVID-19 is called . In order to ensure that the mini-binder would bind to the same RBD that the ACE2 was bound to, AHB2 was designed by looking at the specific sequence of ACE2 to find the alpha-helix that makes interactions with the spike receptor binding domain. This design process is referred to as the Rosetta Blueprint protein design. Figure 2 shows the RBD trimer with one part of ACE2 being used for the reference alpha helix to create AHB2 [5].
As the AHB2 inhibitors were tested and found to be effective, it was then time to manipulate the mini-binders to create a more effective vaccine. A rotamer interaction field docking method with in silico mini-proteins was used with a scaffold library to generate binders to more distinct regions of the RBD surface [5]. This method is known as the de novo protein design and it is how the and mini-binders were created. Figure 3 shows the different LCBs pulled from the scaffold library to create the different LCB inhibitors.
Stability
One of the most important findings with these De Novo proteins is their high stability, allowing for less delicate forms of administration. Additionally, it was found that the Rosetta built minibinder had a lower thermal stability than the completely De Novo proteins. Looking at the , there are only 4 key internal residues significantly contributing to stability, and they are not oriented directly towards the center of the protein for optimal interaction. Comparatively, the and had 5 key internal residues more centrally directed contributing to stability[5].
Binding Site and Interactions
In order to best target the RBD of the spike protein, the minibinders reveal a wide range of interactions to compete with .
The first design method, Rosetta, created AHB2 based on the single interacting helix of ACE2. With , we see two alpha helices mimicking ACE2. Hydrogen bonding interactions between N36, D11, K43, E41, and E30 of the minibinder interact with residues K417, R403, Y449, Q493, and N487, respectively, in the spike protein.

Figure 4: The sequence differences between ACE2, AHB2, LCB1 and LCB3.
De novo designed proteins, as discussed previously, focused on computational design to determine residues best able to interact with the spike protein. We will focus on LCB1 and LCB3. reveals hydrogen bonding between D30 of the minibinder and both K417 and R403 of the spike protein, in addition to D17 and R14 of the minibinder interacting with Q493 of the spike protein. Similarly, reveals hydrogen bonding between D11 of the minibinder to K417 and R403 of the spike protein[5].
Within all four binding sites, we see two conserved residues throughout: K417 and Q493. This finding reveals the importance of these residues in both binding and stability.
Furthermore, an interesting difference between the two design methods is the location of hydrogen bonding interactions. In AHB2, similar to ACE2, we see interactions spanning the entirety of the helices in contact with the spike protein. De novo design, on the other hand, reveals the main hydrogen bonding interactions occurring within the center residues of the helices.
Figure 4 shows the sequence comparison between the interacting helix of ACE2 and the helices of the three minibinders.
Conclusion
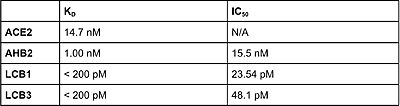
Figure 5: The binding affinity values (Kd) and the half maximum inhibitory concentration values (IC50) are shown for ACE2, AHB2, LCB1 and LCB3
[5].
All in all, minibinders designed to inhibit binding of ACE2 to the spike protein have revealed promising potential. The inhibitors LCB1 and LCB3 were shown to be the most effective at inhibiting the virus by having the highest affinity for the RBD, and as well as the highest neutralization effect as seen in figure 5 [5]. While AHB2 wasn't as effective as LCB1 and LCB3 at neutralizing the virus, it still showed results of being an effective vaccine, as it still had a higher binding affinity to the RBD compared to ACE2. All three inhibitors show more advantages as a vaccine compared to antibodies due to their small size, stability, and their ability to be quickly modified [5]. The use of mini-inhibitors through the de novo protein design has also shown results in being able to control cell function and detecting protein activity within the cell [6].
The limitation behind the design of AHB2 was that it was specifically made from ACE2, making it an inhibitor that can't be universally used to fight other viruses. The biggest limitation regarding the de novo designed inhibitors is identifying the residues that will increase affinity to the spike protein[5].





