Introduction
Isocitrate dehydrogenase (IDH) is an enzyme that is used during the third step of the citric acid cycle. This biological reaction is an essential process that is used to create molecules that are used for cellular energy. In this step it catalyzes the oxidative decarboxylation of isocitrate meaning that CO2 is released from the isocitrate. In addition coenzyme NAD+ is converted to an NADH. This reaction results in an alpha-ketoglutarate molecule which is then moved on to the forth step of the citric acid cycle. In Escherichia coli the IDH is regulated by phosphorylation which is catalyzed by IDH kinase/phosphatase (IDHK/P). Human IDH1 is cytoplasmic while IDH2 is mitochondrial. See also:
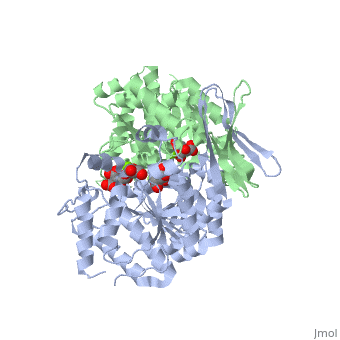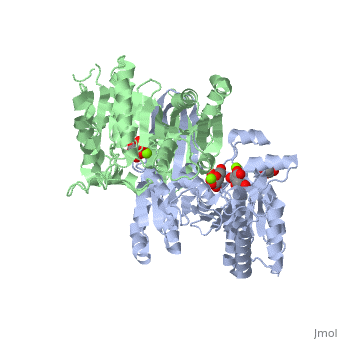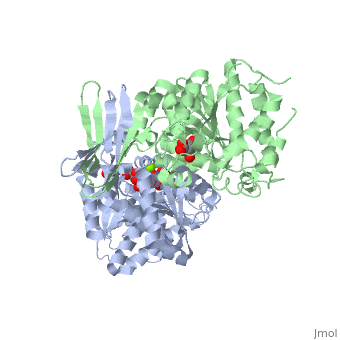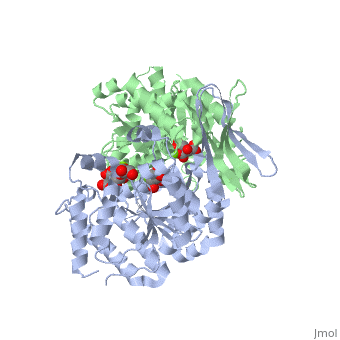
Structural Analysis
Isocitrate dehydrogenase is SCOP classified as an alpha beta structure. Its secondary composition consists of mainly alpha helices and beta sheets which are arranged into three layer alpha beta alpha . The entire protein consists of two side by side sandwich structures that face opposite directions. This then causes the proteins two active sites to face opposite directions. These two groups make up the A and B subunits of isocitrate dehydrogenase.In human isocitrate dehydrogenase there are 4 subunits.
The Active Site
The active site of Isocitrate dehydrogenase binds the NAD+ or an NADP+ molecule as well as an Mg2+ or Ca2+(). This metal ion seems to be essential for catalysis[1]. Side chains of Asp 279 form hydrogen bonds with Ser94 (Human isocitrate dehydrogenase). Isocitrate is able to bind to the active site using about 8 amino acids like tyrosine, serine, asparagine, arginine, arginine, arginine, tyrosine, and lysine[2]. Across species these are not perfectly conserved but they are replaced with residues of similar properties and also are found in similar areas of the protein. Below is a picture of Porcine Active site with all of its residuals and ligands.
Mg+ ion (red) next to the ligand which is surrounded by the residues of the active site[3].
Function
Isocitrate dehydrogenase is a digestive enzyme that is used in the citric acid cycle. Its main function is to catalyze the oxidative decarboxylation of isocitrate into alpha-ketoglutarate. Human isocitrate dehydrogenase is regulation is not fully understood however, it is known that NADP and Ca2+ bind in the active site to create three different conformations. These conformations form in the active site and are as follows: a loop is form in the inactive enzyme, a partially unraveled alpha helix in the semi open form, and a alpha helix in the active form[4]. Bacterial isocitrate dehydrogenase uses phosphorylation for regulation. The Ser94 residue undergoes reversible phosphorylation causing structural changes in the active site which hinders the catalytic function of the enzyme [5].
Mechanism
The mechanism described is for porcine isocitrate dehydrogenase because is is better understood than the human mechanism. The is converted to alpha-ketoglutarate. In the first step the alcohol group
off the alpha-carbon is deprotonated by the Tyr residue. The electrons push to the oxygen atom to form a double bond (keytone). The remaining
alpha carbon hydrogen is removed using NAD+/NADP+ as an electron accepting cofactor. A carboxyl group pushes electrons down so an oxygen steals a nearby proton off a Lysine amino acid. The Tyr deprotanates the carboxylic acid resulting in electron pushing that ejects a CO2. The two negatively charged oxygen's on the other side of the molecule are stabilized by the Mn2+. The double bond that was formed between the alpha and beta carbon removes a proton from the Tyr residue and the oxygen returns the a keytone and the alpha-ketoglutarate is formed. This is illustrated in the figure below.
The mechanism by which isocitrate is converted to alpha-ketoglutarate [6]
Inhibition
(1t01).
This step an irreversible reaction. It must be carefully regulated to avoid depletion of isocitrate in the body. The reaction is started by substrate availability which are: isocitrate,Mg2+, NAD+ or NADP+. If these are not present than the process will not carry forward. The reaction is inhibited by the removal of NADH from the presence of the citric acid cycle. The product is also inhibited by ATP feedback. This feedback inhibition is a competitive inhibitor. Since the citric acid cycle is used to produce energy molecules (ATP), an abundance of product will shut down the cycle.
Involvement in Cancer
Mutations in Isocitrate Dehdryogenase isotypes, IDH1 and IDH2 have recently been discovered in CNS cancers like Gliomas, and a number of types of leukemia, including Acute Myeloid Leukemia. This discovery has been extended to prostate cancer, colon cancer, and thyroid cancer as well since 2009. Mutations to residue Arg 132 are the most common cancer inducing mutation. . Instead of producing alpha-ketoglutarate, this mutant produces 2-hydroxyglutarate.
There are two competing theories as to how this mutation to IDH is cancer inducing. The first theory stipulates that the 2-hydroxyglutarate (2HG) produced inhibits proline hydroxylase (PHD). PHD is responsible for telling the cell if there is ample oxygen present for oxydative phosphorylation by hydroxylating Hypoxia Inducing Factor (HIF). When 2HG inhibits PHD, HIF is transported to the nucleus where it is believed to abberantly activate gene transcription, leading to cancerous conditions.
The alternative theory, which is recently gainging significant support, states that IDHs hypermethylate DNA sites which code for proteins assoicated with cell differentiation, repressing these genes. Mutant IDHs however produce 2HG which is a competitive inhibitor of TET2, a protein which hydroxylates methylated cytosines, effectively resulting in demthylation. With TET2 inhibited, the cell differentiation inducing genes remain inactive resulting in undifferentiated cancer cells. [7]
Additional Resources
3D Structures of Isocitrate dehydrogenase
Isocitrate dehydrogenase 3D structures