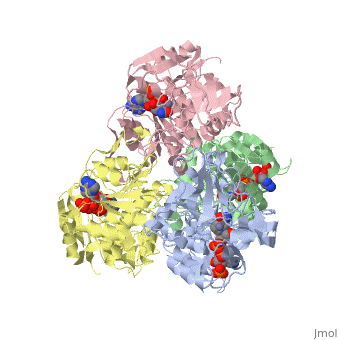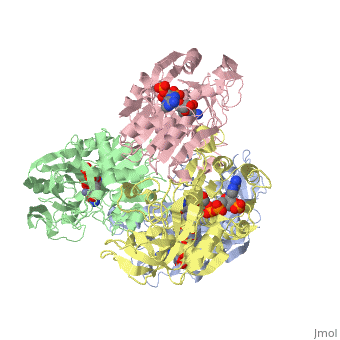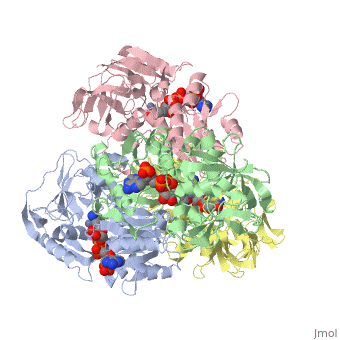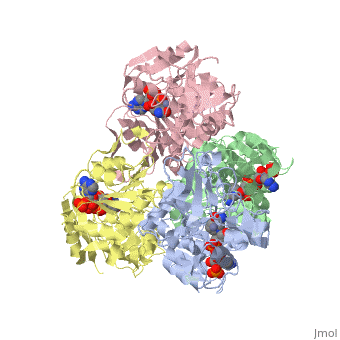
has an extraordinary ability to remain folded in extreme temperatures - up to 95 degrees celsius! How an enzyme can withstand these extreme temperatures depends on enthalpic and entropic strategies, as seen from the GIbb's equation: ∆G = ∆H - T∆S; Where ∆G is negative, the products form spontaneously, which in our context means proteins will fold spontaneously. From the equation is is clear that to maintain a value of ∆G, the enzyme has two available paths. It can decrease ∆H, or it can increase ∆S. TbADH does both.
Professor Yigal Burstein and colleagues, at the Weizmann Institute of Science, have demonstrated two strategies, through experiments using chimers. First, through an in the interface of the monomers, the enzyme has a decreased enthalpy in the folded state, meaning it is more stable - ∆H decreases. TbADH also has
at strategic positions. Because proline undergoes fewer configurational changes in an unfolded protein, therefore there is less restriction of its movement in the folded state, which means the loss in entropy is less, and therefore ∆S increases.
Through studies like Prof. Burstein's, researchers are gaining insight into how proteins play by the rules of thermodynamics. Any insight into the physics of protein stability has that satisfying feeling that accompanies successful reductive explanation.