This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
JMS/sandbox6
From Proteopedia
Your Heading Here (maybe something like 'Structure')
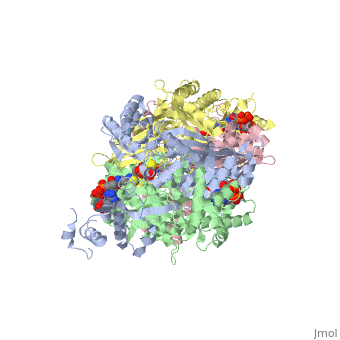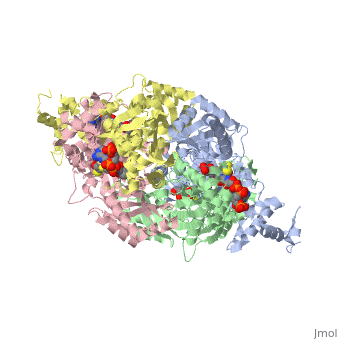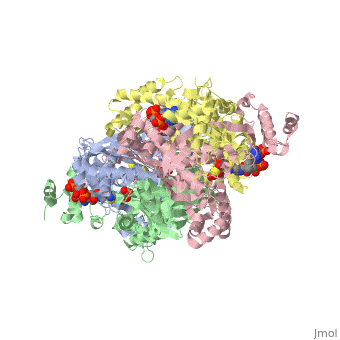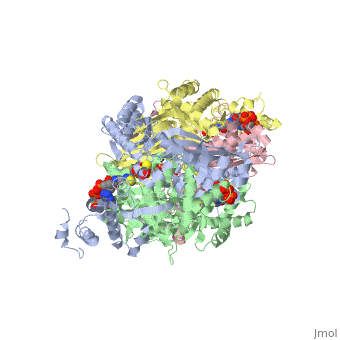
Anything in this section will appear adjacent to the 3D structure and will be scrollable. Extremophiles must stabilize their protein synthesisLife exists in some extreme environments. To cope with off the charts chemical and physical stress, organisms have adopted strategies ranging from physiological to behavioral. But almost always its not just the organism as a whole that must deal with the situation, but all its molecules. Proteins must devise ways to remain structurally sound.
Too high temperature or too much salt stresses protein structuresTwo stresses, high temperature and variable amounts of salt concentration place particular stresses on proteins. At the Weizmann Institute of Science, two labs have pursued projects to resolve a protein's acclimation to high temperature and to wide-ranging salt conditions.
Prof. Burstein at the Weizmann Institute studied molecular adaptation to temperature stressesFirstly, a bit of thermodynamic background, as it lies at the foundation of environmental challenges to molecules such as proteins. Stresses must be interpreted into the language of thermodynamics. To deal with high temperatures, an organism can either increase the disorder, or entropy, of the folded state, that is, reduce the increase in order upon folding; and/or an organism can increase the stability of the folded protein, which corresponds to a lowered enthalpy in the folded state. The equation: ∆G – ∆H-T∆S, expresses this notion. Where ∆G, the change in Gibbs free energy/ the energy available to do work, is negative, the reaction from unfolded state to folded state, that is, the protein folding, occurs spontaneously. ∆H is the change in enthalpy from unfolded to folded, and ∆S is the similar change in entropy, upon folding. T stands for the temperature, in Kalvin units. As seen from this equation, where T increases, as is the case for the condition of high temperature, the protein can either increase the value of ∆S or decrease ∆H. Studying an enzyme from a bacteria adapted to living in extreme environment, Prof Burstein showed that its protein do both. They increase ∆S and decrease ∆H. By incorporating more prolines, the protein increases ∆S. Because proline amino acids have relatively fewer degrees of freedom in their side chain compared to other amino acids, therefore restriction of its side chain configuration upon folding is less energetically costly. The enzyme they studied also decreases the value of ∆H, by increasing the stability of the enzyme, through an electrostatic ion network, involving four amino acids from each of four monomers: three from one monomer and one from and adjacent monomer. These two strategies are apparent through comparison with enzymes which, since they are not adapted to high temperature, do not need these two strategies to increase stability; indeed, should not decrease ∆G, for reasons of dynamics. Prof Burstein's work does the important job of resolving theoretical thermodynamics expectations, with hard molecular experimental data.
Prof. Joel Sussman and Dr. Adi Zamir at the Weizmann Institute studied molecular adaptations to varying salt concentrationsComplementing this work on extreme thermopiles, Prof. Joel Sussman together with Dr. Adi Zamir, and their labs, also at the Weizmann Institute of Science, have studied the bacteria Dunaliella salina, which lives on the shores of the Dead Sea, where it is sometimes exposed to high salt, but critically, it is also sometimes exposed to normal salt concentrations. Sussman and his team of researchers asked how the enzymes lying on the outside of the bacteria membrane –exposed to varying concentrations of salt – maintain their shape – during the different conditions, ranging from normal to extreme. They compared the enzymes from D. salina to similar enzymes, from bacteria exposed to alternative environments. Comparing these enzymes to enzymes found in bacteria always inhabiting normal salt concentration, and to enzymes found in bacteria which are always exposed to high salt concentrations, they found that the enzymes of D. salina exhibit an intermediate property. Apparently, decreasing the concentration, or density, of basic amino acids, particularly lysine, on the exposed surface of the enzyme, protect the enzyme from salt molecules. Those enzymes always exposed to fair condition have a relatively constant relative and absolute amount of amino acids, and are used as a reference. Those enzymes which must constantly cope with high concentration of salt, have a decreased number of basic amino acids on the surface and an increased number of acidic amino acids, and are hence (relative to the reference) strongly negative. In contrast, those enzymes which can survive/fold in high or normal salt condition have less basic amino acids, but the same number of acidic amino acids, and are therefore more negative overall than regular enzymes, but less negative than halophilic enzymes. While this change in negative density is a marked indicator of enzyme suitability for different conditions, how this difference in charge density relates to the theoretical properties of thermodynamics of protein folding is an open question. Proteopedians reading this, feel encouraged to add your (sourced) ideas.
| |||||||||||