The impact of molecular variants, crystallization conditions and the space group on ligand-protein complexes: A case study on bacterial phosphotriesterase
Orly Dym, Nidhi Aggarwal, Yacov Ashani, Haim Leader, Shira Albeck, Tamar Unger, Shelly Hamer Rogotner, Israel Silman, Dan S. Tawfik and Joel L. Sussman [1]
Molecular Tour
A bacterial phosphotriesterase was employed as an experimental paradigm for examining the effects of multiple factors, such as the molecular constructs, the ligands used during protein expression and purification, crystallization conditions, and space group, on the visualization of molecular complexes of ligands with a target enzyme. In this case, the ligands used were organophosphates that are fragments of the nerve agents and insecticides on which the enzyme acts as a bioscavenger. Twelve crystal structures of various phosphotriesterase constructs obtained by directed evolution were analyzed, with resolutions up to 1.38 Å. Both apo forms and holo forms, complexed with the organophosphate ligands, were studied. Crystals obtained from three different crystallization conditions, crystallized in four space groups, with and without N-terminal tags, were utilized to investigate the impact of these factors on visualizing the organophosphate complexes of the enzyme. The study revealed that tags used for protein expression can lodge in the active site and hinder ligand binding. Furthermore, the space group in which the protein crystallizes can significantly impact the visualization of bound ligands. It was also observed that the crystallization precipitants can compete with, and even preclude, ligand binding, leading to false positives or to incorrect identification of lead drug candidates. One of the co-crystallization conditions enabled us to define the spaces that accommodate the substituents attached to the P atom of several products of organophosphate substrates after detachment of the leaving group. The crystal structures of the complexes of phosphotriesterase with the organophosphate products reveal similar short interaction distances of the two partially charged oxygens of the P-O bonds with the exposed β-Zn2+ ion and the buried α-Zn2+. This suggests that both Zn2+ ions have a role in stabilizing the transition state for substrate hydrolysis. (PDB-ID 1hzy). The (β/α)8 TIM barrel fold is shown as a cartoon, with helices in red, sheets in yellow, coils in green, the α- (buried) and β- (exposed) Zn2+ ions as magenta spheres, and a single bridging water shown as a cyan sphere. The six residues that bind to the two Zn2+ ions are shown as ball-and-stick figures, with carbon atoms coloured yellow, nitrogens blue, and oxygens red. The N- and C-terminal residues are labelled N and C, respectively. . The buried α-Zn2+ is directly bound to H55, H57, and D301, while the exposed β-Zn2+ is bound to H201 and H230. The carbamate functional group bound to K169 interacts with both Zn2+ ions. Colouring is as in previous scene.
The (PDB-ID
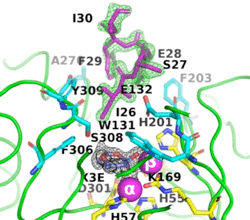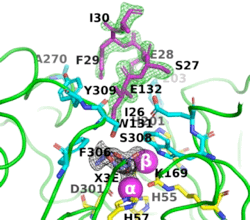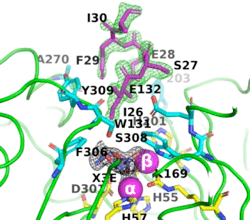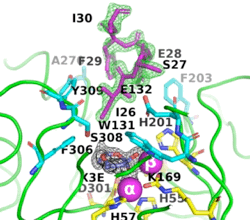
8p7i) shows the octapeptide tag on subunit B penetrating the active-site region of the symmetry-related subunit A. The tag is shown in magenta. The active-site residues of the symmetrically related chain A are shown in yellow, with those residues within 5 Å of the tag shown in cyan. The cyclic compound, X3E, presumably carried over from the protein expression and purification process, is seen in the active site. The two Zn
+2 ions are shown as magenta spheres.
The image on the left shows the electron density (in green) of an omit map with the octapeptide omitted (contoured at 3 sigma). The cyclic compound, X3E, presumably carried over from the protein expression and purification process, is seen in the active site, and the electron density (in black) corresponds to a 2Fo-Fc map (contoured at 1 sigma). It is clear that the particular crystallization conditions and space group can have an enormous impact on whether a ligand binds to a protein.
The (PDB-ID 8p7u), shows the active-site residues in yellow. the two Zn+2 ions as magenta spheres.
(PDB-ID 8p7u). The six residues that bind to the two Zn2+ ions are shown as ball-and-stick figures, with carbons coloured yellow, nitrogens blue, oxygens red, and phosphorus atoms orange. The interatomic distances observed between the oxygens of the P-O in all OP acid products are 1.9-2.0 Å.
Overall, this study provides valuable insights into the challenges and considerations involved in studying the crystal structures of ligand-protein complexes, highlighting the importance of careful experimental design and rigorous data analysis in ensuring the accuracy and reliability of the resulting PTE-OP structures obtained.
References
- ↑ Dym O, Aggarwal N, Ashani Y, Leader H, Albeck S, Unger T, Hamer-Rogotner S, Silman I, Tawfik DS, Sussman JL. The impact of molecular variants, crystallization conditions and the space group on ligand-protein complexes: a case study on bacterial phosphotriesterase. Acta Crystallogr D Struct Biol. 2023 Nov 1;79(Pt 11):992-1009. PMID:37860961 doi:10.1107/S2059798323007672



