Kratom
From Proteopedia
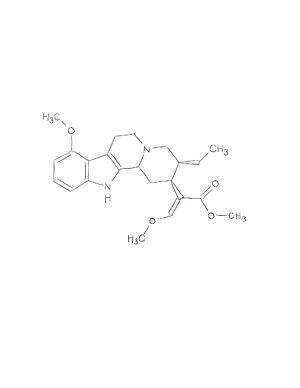
Mitragynine and 7-hydroxymitragynine
Mitragyna speciosa is a plant species that grows in forests at low altitudes indigenous to Thailand, Malaysia, and Myanmar. Mitragyna speciosa is otherwise known as Kratom. In indigenous lands, Kratom is generally taken as a dried powder mixed with a liquid, brewed as a tea directly from its leaves, chewed on, and in some cases smoked in order to reduce pain, help with opioid withdrawal symptoms, diarrhea, cough, and among other medical side effects. Over twenty-five different alkaloids have been able to be isolated from Kratom leaves, two of them being mitragynine and 7-hydroxymitragynine. Mitragynine and 7-hydroxymitragynine are 398.5 g/mol and 414.5 g/mol respectively and are hydrophobic. In Kratom, 7-hydroxymitragynine consists of only approximately 2% of the total molecular makeup. While the majority of Kratom consists of Mitragynine (approximately 66.2%), mitragynine is easily converted to 7-hydroxymitragynine in vivo[1]. The compound 7-hydroxymitragynine is the primary opioid agonist and analgesic present in the plant although mitragynine has been shown to exhibit binding as well. With the ability for mitragynine and 7-hydroxymitragynine to bind the u-opioid receptor (where most other opioids and analgesics bind) there is a potential new source of medicine. For sake of simplicity- the remainder of this page will use mitragynine and 7-hydroxymitragynine interchangeably.
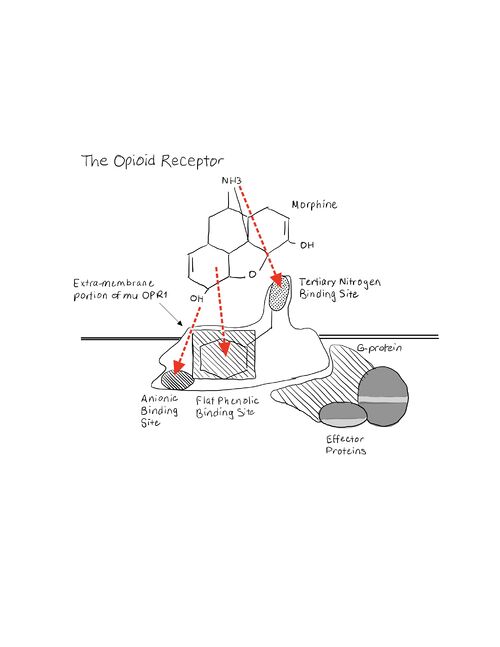
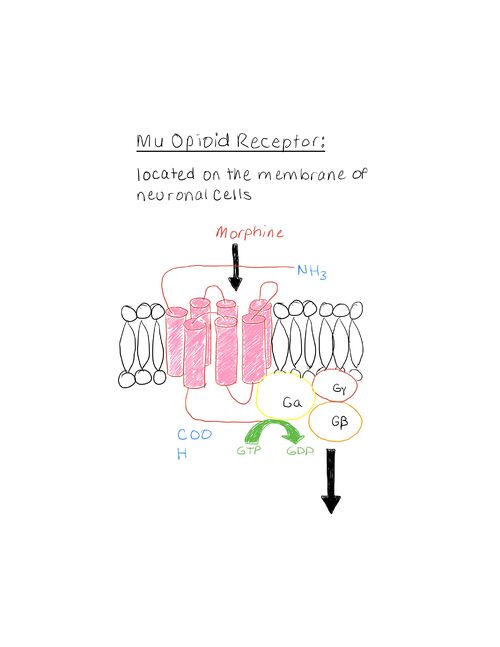
Current ResearchA study done in April of 2015 on the Pharmacokinetics of Mitragynine in Humans[2] showed promising results for Kratom to become a new substitute for opioid pain medication. While the sample size was low, the study observed ten males who chronically took Kratom without any adverse reactions. Dosing of the oral extract was controlled and vital signs within each individual were observed as well. Mitragynine was found to have a terminal half-life of approximately 24 hours and a time to reach maximum concentration in the plasma of approximately 50 minutes. Mitragynine’s ability to rapidly be absorbed into the bloodstream and take a day to be excreted exhibits a two-compartment model. The study also observed that concentrations of mitragynine were very low in the urine thus indicating low use of the kidneys for excretion. Researchers were given no evidence of emergent side effects or emergent vital signs. Most opioids bind to the opioid receptors within the central nervous system thus they have the ability to cross the blood-brain barrier. The three most notable opioid receptors are the mu, delta, and kappa. A very exciting study finally crystallized . The interactions between drugs and opioid receptors are not fully understood; though this is the case research in this field has made large strides in the right direction. By binding to opioid receptors neurotransmission to the brain is blocked. A study done titled “Pharmacokinetics of mitragynine, a major analgesic alkaloid in kratom (Mitragyna speciosa): A systematic review”[3] found that not only does mitragynine passively cross the blood-brain barrier it also binds to the mu, delta, and kappa opioid receptors. Many know of the common opioid receptor agonist morphine. As morphine enters the an amine group orients across from an aspartic acid residue. The negatively charged amino acid and the positively charged amine group are able to form an ionic interaction. This interaction is not just unique to the mu-opioid receptor- it is conserved throughout all others as well. This illustrates the importance for this residue to be present in order for ligand and protein binding to occur. The ring structure of morphine allows orientation across from a histidine residue. Opioid receptors are made up of primarily membrane-spanning alpha-helices coupled to a G-protein. When opioid binding occurs it induces a conformational loop change on the inner side of the cell membrane activating and releasing the G-protein. This G-protein sometimes recruits another small protein called beta-arrestin. B-arrestin recruitment is one of the major reasons behind an opioid’s lethal side effects such as respiratory distress and desensitization which may lead to addiction. The alpha subunit of the G-protein that is released inhibits adenylyl cyclase while the beta and gamma subunits inhibit calcium channels and activate potassium channels[4]. By inactivating adenylyl cyclase the drug is inhibiting cAMP production and thus turning off many extra-cellular signals. In addition, inhibition of calcium channels and activation of potassium channels creates a hyperpolarized environment[5]. This occurs when a cells’ membrane potential becomes more negative and thus a higher stimulus is required to reach an action potential threshold. This process ends up inhibiting action potentials. Mitragynine has a methyl-ether group that interacts with V143 and C217 present on the opioid receptor. Mitragynine has a secondary and tertiary amine that is capable of interacting with the important D147 residue as well as a ring structure that is capable of orienting across from the histidine residue. A recent study published in August of 2020 on mitragynine and 7-hydroxymitragynine binding to the mu-opioid receptor[6][7] investigated if and how mitragynine was able to enter the active site. The study discovered that not only does mitragynine bind in the active site but it also activates the G-protein pathway. The interesting thing about the activation pathway is that the majority of the time it does not recruit beta-arrestin. Unfortunately, Cytochrome P450 metabolic enzymes have been shown to involve with the metabolism of mitragynine. The enzymes seem to be inhibited by mitragynine and therefore raise concern for adverse drug reactions. Cytochromes P450 are heme-containing enzymes that oxidize steroids, fatty acids, and xenobiotics. These enzymes also play a large role in clearing a hefty amount of compounds from the body and aid in hormone synthesis and decay[8].
Clinical SignificanceDue to the low evidence of mitragynine in the urine, this may indicate kratom is not processed through the kidneys, it is therefore hypothesized that patients with impaired renal function may benefit from such a pain killer. The ability for Kratom to be metabolized quickly and remain in the system for nearly a day opens opportunities for it to be used as an effective longer-lasting medication. The fewer side effects observed open the possibility of longer-term treatments for chronic pain. Like other opiates, mitragynine possesses the ability to cross the blood-brain barrier and bind to opioid receptors thus allowing the patient to experience relief from pain. While further research must be done to determine whether mitragynine posses any anti-inflammatory properties, blocking out pain is a solid start. The ability for mitragynine to act on the opioid receptor in a similar fashion to other opiates- activating the G-protein pathway that raises action potential thresholds and inhibiting adenylyl cyclase- all without the recruitment of beta-arrestin allows for the possibility for mitragynine to act as a safer and effective pain killer. Since beta-arrestin is not recruited, respiratory depression is not a concern for patients taking mitragynine as an analgesic. Although this is the case further research is needed in order to determine if Kratom is as mentally and physically addictive as other opioids. In addition, mitragynine was found to be metabolized in the liver thus indicating the possibility of eventual overuse and liver cirrhosis. Currently, research struggles to determine specific dosing, purity, and risk of overdose in humans as animal studies only take research so far[7]. LegalityAccording to the World Health Organization, in 2018 approximately 35.6 million people suffered from a drug use disorder, overwhelmingly opioid-related[9]. Mitragynine shows promising results to reduce opioid abuse. Over the past couple of years, The United States drug enforcement agency, as well as many other countries' governments, tried to enforce an emergency order that scheduled mitragynine and 7-hydroxymitragynine as an illegal substance. Many citizens stood up against this action and the enforcement was withdrawn[7]. This continued to allow for the study of Kratom’s potential risks and benefits without intervention. Governments are encouraging more in-depth research at this point in time; it is needed to bring about approval across many different nations as the substance is not regulated. Though this is the case it remains legal and attainable at local shops and online. With more attention being brought to Kratom hopefully more in-depth research will be completed. References
| ||||||||||||