This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Lauren Ferris/Sandbox 1
From Proteopedia
Contents |
Activated Protein C
|
Introduction
The vitamin K dependent zymogen Protein C is an integral component of the blood coagulation system. The blood coagulation system is composed of two pathways that converge to form the prothrombinase complex (Factor Va-Factor Xa). Thrombin is generated from this complex and catalyzes the formation of the clot by activating Fibrinogen and FXIII. Thrombin will also bind to thrombomodulin and this complex removes 12 peptides(Arg169-Leu170) from the protein, converting it to the active form: Activated Protein C.[1][2][3] Activated Protein C will function as a down-regulator of procoagulant stimuli through the degradation of zymogens and their active counterparts: FV/FVa and FVIII/FVIIIa. [4][5] This ability enables Protein C to modulate the coagulant response, preventing unchecked clotting.
Protein C has also been recognized for its role in anti-inflammatory and cytoprotective signaling responses in endothelial cells. Protein C will bind to an endothelial protein c receptor (EPCR) and then activate protease-activated receptor (PAR-1).[6]
Protein C is synthesized in the liver and circulates the blood at a concentration of 4 ug/mL. The liver conformation is a single polypeptide sequence with an additional 42 amino acids attached at the N-terminus. This structure is known as the pre-pro sequence. Subsequent removal of these peptides as well as Lys156-Arg157 will result in the 62 kD protein that is observed.[7]
Structure
Activated protein C is a spheroid shaped molecule with both alpha helices and beta sheets. The protein contains similar domains to other vitamin K dependent coagulation proteins with a and connected by a di-sulfide bond. The light chain contains a Gla domain and 2 epidermal growth factor domains, while the heavy chain contains an activated peptide domain and a serine protease domain.
The Gla domain contains glutamic acids, which are converted to y-carboxyglutamic acid by a Vitamin K dependent carboxylase. The y-carboxyglutamic acid enables the protein to bind to Ca2+ and undergo a conformational change that enables binding to a phospholipid bilayer. [8][9]
Epidermal Growth Factor Domain 1. This domain is proximal to the n-terminal domain. Activated Protein C EGF-1 contains a unique seven residue insert and an extra-disulfide compared to other EGF domains in other proteins. This gives the protein a total of four di-sulfide bridges existing between Cys50-69, Cys59-64,and Cys63-78 and 80-89.[10] Three major loops are formed from these disulfides. The first loop is actually composed of two loops with Cys 50-69 forming a larger loop and Cys 59-64 forming a smaller loop inside. The terminal residues of EGF1 83-85 form an anti-parallel pleated sheet with residues 91-93 to connect to EGF2.[10]
Epidermal Growth Factor Domain 2. This domain is sometimes referenced as the c-terminal EGF-2, although it is adjacent to EGF1. EGF-2 is a fairly standard EGF domain containing disulfide bridges that form loops.[10] Activated protein C contains both a heavy and light chain. The light chain includes residues from the N-terminus of the protein to several residues after the C terminus of the EGF-2 domain.
Serine Protease Domain. The serine protease domain is made up of two six stranded antiparallel beta barrel domains that cross through the core. The barrel domains also form loops that help to comprise the surface of this domain. The protein contains structural elements such as a buried ion pair, an active site between barrel domains, surface helices, di-sulfides, and a Ca2+ binding loop. Unique to Protein C include n-terminal leucine next to the active site.[10]
Surface Structure. A cluster of positive charges have been observed. Based on homology, this region is thought to be involved with substrate and inhibitor interactions with the protein. An insertion lending to a helix forming, and a loop at the edge of the catalytic site also exist.[10]
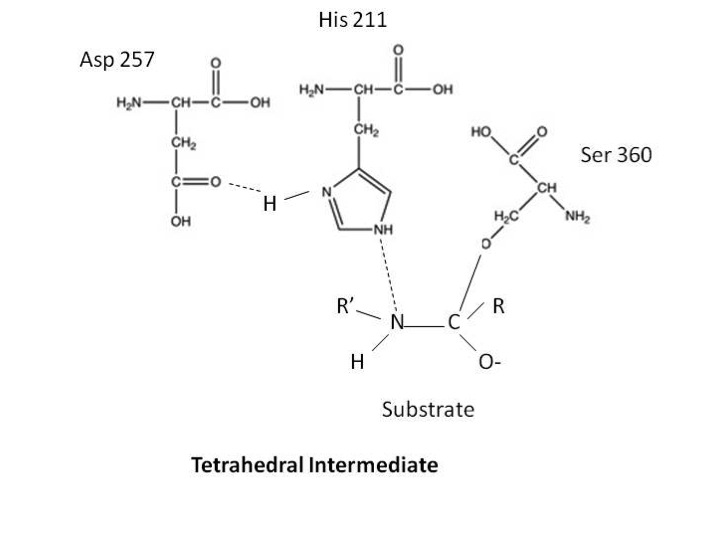
Active Site Structure. Active site contains Histidine 211, Aspartic Acid 257, and Serine 360. Structural hindrance preventing binding to the active site is a 60 residue loop. [10] Other loops also help to form a deep depression near the active site.
Mechanism
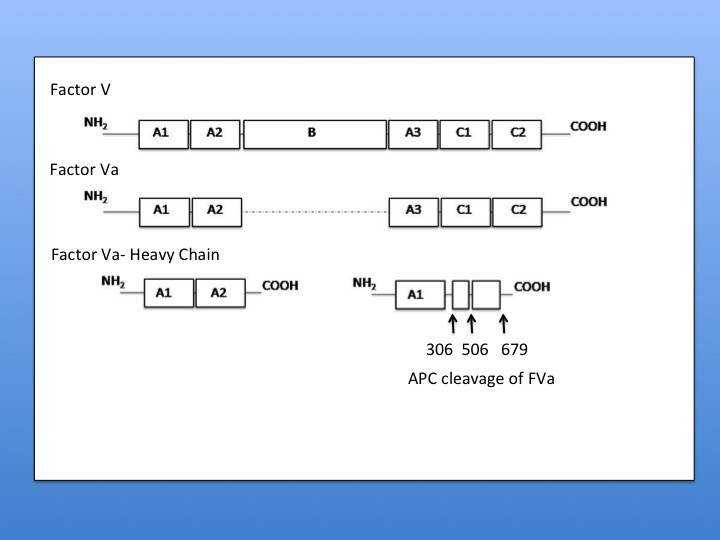
Activated Protein C has been shown to be a highly functional enzyme catalyzing reactions under a variety of conditions. Activated Protein C will also degrade several blood coagulation zymogens and their activated forms: Factor V/FactorVa and Factor VIII/Factor VIIIa to modulate the procoagulant response. [4][5] In order for activated protein C to degrade Factor Va phospholipid bilayer must be associated with the substrate. Provided that a membrane-associated Factor V is present, activated protein C will cleave Factor V at Arg 306 then Arg506 followed by Arg 679 and Lys 994. [11] In the case of Factor Va cleavage, there is no cleavage of the light chain in the absence of a phospholipid bilayer. Under these conditions activated protein C will cleave the heavy chain at Arg506 and Arg 679 to give a partially active cofactor. [11] When the lipid bilayer is present, Activated Protein C will cleave at Arg 506, then Arg 306, and then Arg 679. Cleavage at Arg 506 is required for exposure of sites at 306 and 679. Since cleavage at Arg 306 is sufficient for inactivation, it can be concluded that the light chain binds to the phospholipid bilayer making this cleavage site available to APC once 506 is gone. [11]
Research has suggested that the degradation of these blood coagulation proteins is because activated protein C contains a catalytic triad, as seen in serine proteases. Early experiments treated activated protein C with tritiated diisopropylphosphorofluoridate. (Diisopropylphosphorofluoridate is a suicide inhibitor of serine proteases as it will form a covalent bond with the serine in the catalytic triad). Activated Protein C was able to bind to this substrate. Further characterization of this reaction revealed that the catalytic site was in the heavy chain and utilizes residues Asp257, His211, and Ser360 to cleave factors FV and FVIII and their activated forms. [8][9] [12][10]
The implications of this catalytic triad are that these residues are within a pocket of the enzyme. The enzyme will bind to a substrate and form a tetrahedral intermediate by using the serine in nucleophilic attack of a peptide bond in the substrate. The serine is able to attack the peptide after the hisitidine in the triad obtains a proton from serine’s R-group. The resulting tetrahedral intermediate is said to ressemble the transition state of the reaction. This reaction also forces the carbonyl oxygen of the substrate deeper into a hole in the active site that was previously empty. This region of the is known as the oxyanion hole and it is here that the oxygen will form two hydrogen bonds with the enzyme. This will also enable the formantion of hydrogen bonds to the enzyme on the amino side of the substrate peptide bond. These extra hydrogen bonds are not the only force helping to stabilize the transition state. The formation of low-barrier hydrogen bonds is thought to help stabilize the tetrahedral intermediate. Low Barrier hydrogen bonds are short and strong hydrogen bonds that form in nonaqueous regions. They are thought to form between the histidine and aspartic acid in the catalytic triad based on distance between residues and the nonaqueous environment of the triad.
An acyl-enzyme intermediate will form as the tetrahedral intermediate decomposes. The acyl-enzyme intermediate forms as N3 from histidine donates a proton in a general acid catalysis. The proton donation enable the amine to leave the active site and enables the N3 of histidine to bind to a hydrogen in water. The carbonyl of the peptide substrate binds to the oxygen of water. Hydrolytic cleavage than produces a second tetrahedral intermediate. Finally the enzyme is regenerated when the water acts as an attacking nucleophile.
The crystal structure of activate protein c supports the serine protease mechanism. It also, as mentioned above, shows a deep depression by the active site enabling semi-restricted access. [10]
Further Experimentation has also investigated whether the cleavage of Factor Va or the dissociation of the cleaved peptides are responsible for protein inactivation. This was done through generation of intramolecular disulfide bonds, that would keep peptides from dissociating after cleavage. Results determined that the cleavage itself was sufficient for substrate inhibition. [13]
Activate protein C functions optimally in the presence of a phospholipid bilayer and with its cofactor protein S, as protein S positions the substrate and activated protein C . [14]
Related Proteins
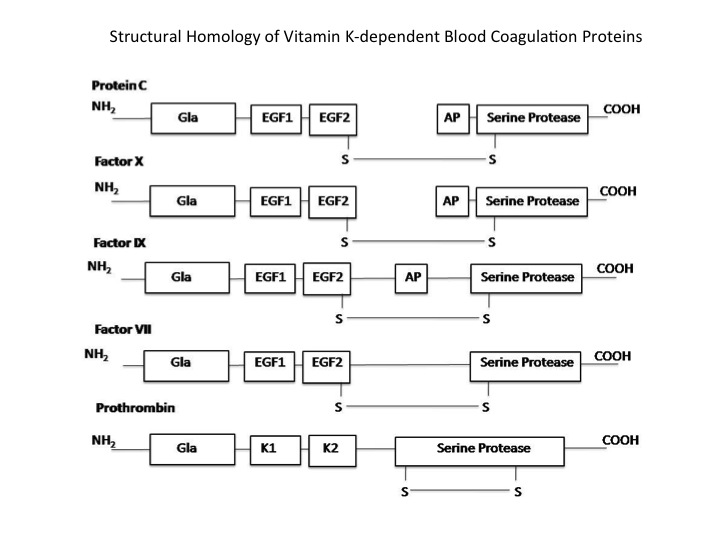
In general, protein C has a high degree of homology to other vitamin k dependent serine proteases. These Gla domain containing proteins include but are not limited to blood coagulation proteins. Non-blood coagulation proteins include trypsin and chymotrypsin. Coagulation proteins with a high degree of homology include prothrombin, Factor VII, Factor IX, and Factor X. Protein S and Protein Z are also fairly homologous, but they are not serine proteases.[1][2][3] Despite the similarity in structure, the enzymes retain a high degree of specificity for their substrates, perhaps due to the nonhomologous surface loops that are away from the substrate binding pocket.
Links to Available Structures
The Protein Data Bank has Human Acitvated Protein C - 1AUT
The Protein Data Bank has Human Activated Protein C complexed with PPACK - 3F6U
Clinical Relevance
Protein C has also been shown to have clinical relevance. Protein C deficiency has been observed in patients and is thought to contribute to a higher incidence of thrombotic events. FV Leiden mutation, is the mutation of the Arg506 to Gln506 preventing FVa from degradation and inactivation by Activated Protein C. Individuals with the FV Leiden mutation also have a higher incidence of thrombosis.
Interesting Facts
Copperhead snake venom is thought to contain protein c activators.
References and Notes
- ↑ 1.0 1.1 Esmon CT. The regulation of natural anticoagulant pathways. Science. 1987 Mar 13;235(4794):1348-52. PMID:3029867
- ↑ 2.0 2.1 Esmon CT. Molecular events that control the protein C anticoagulant pathway. Thromb Haemost. 1993 Jul 1;70(1):29-35. PMID:8236111
- ↑ 3.0 3.1 Stenflo J. Structure-function relationships of epidermal growth factor modules in vitamin K-dependent clotting factors. Blood. 1991 Oct 1;78(7):1637-51. PMID:1912552
- ↑ 4.0 4.1 Suzuki K, Stenflo J, Dahlback B, Teodorsson B. Inactivation of human coagulation factor V by activated protein C. J Biol Chem. 1983 Feb 10;258(3):1914-20. PMID:6687387
- ↑ 5.0 5.1 Fay PJ, Smudzin TM, Walker FJ. Activated protein C-catalyzed inactivation of human factor VIII and factor VIIIa. Identification of cleavage sites and correlation of proteolysis with cofactor activity. J Biol Chem. 1991 Oct 25;266(30):20139-45. PMID:1939075
- ↑ Rezaie AR. Regulation of the protein C anticoagulant and antiinflammatory pathways. Curr Med Chem. 2010;17(19):2059-69. PMID:20423310
- ↑ Brummel-Ziedin K, Orfeo T, Jenny NS, Everse SJ, and Mann KG. Wintrobe's Clinical Hematology. (2004, 11th ed) Chapter 21. Lippincott Williams and Wilkins ISBN:0781736501
- ↑ 8.0 8.1 Stenflo J. A new vitamin K-dependent protein. Purification from bovine plasma and preliminary characterization. J Biol Chem. 1976 Jan 25;251(2):355-63. PMID:1245477
- ↑ 9.0 9.1 Esmon CT, Stenflo J, Suttie JW. A new vitamin K-dependent protein. A phospholipid-binding zymogen of a serine esterase. J Biol Chem. 1976 May 25;251(10):3052-6. PMID:1270437
- ↑ 10.0 10.1 10.2 10.3 10.4 10.5 10.6 10.7 Mather T, Oganessyan V, Hof P, Huber R, Foundling S, Esmon C, Bode W. The 2.8 A crystal structure of Gla-domainless activated protein C. EMBO J. 1996 Dec 16;15(24):6822-31. PMID:9003757
- ↑ 11.0 11.1 11.2 Kalafatis M, Rand MD, Mann KG. The mechanism of inactivation of human factor V and human factor Va by activated protein C. J Biol Chem. 1994 Dec 16;269(50):31869-80. PMID:7989361
- ↑ Kisiel W, Ericsson LH, Davie EW. Proteolytic activation of protein C from bovine plasma. Biochemistry. 1976 Nov 2;15(22):4893-900. PMID:990250
- ↑ Gale AJ, Xu X, Pellequer JL, Getzoff ED, Griffin JH. Interdomain engineered disulfide bond permitting elucidation of mechanisms of inactivation of coagulation factor Va by activated protein C. Protein Sci. 2002 Sep;11(9):2091-101. PMID:12192065 doi:10.1110/ps.0210002
- ↑ Yegneswaran S, Wood GM, Esmon CT, Johnson AE. Protein S alters the active site location of activated protein C above the membrane surface. A fluorescence resonance energy transfer study of topography. J Biol Chem. 1997 Oct 3;272(40):25013-21. PMID:9312108