Introduction
Magainin are a class of antimicrobial peptides (AMPs) found in the African clawed frog Xenopus Laevis.
AMPs consists of 10-50 amino acids, and are produced by Eukaryotes, as part of their defence mechanism from bacteria. For informatoin about AMPs you can visit the Proteopedia page Antimicrobial peptides
Magainin 1 and 2 were discovered by Dr. Michael Zasloff and first reported in 1987. They have an alpha helix structure, and are water soluble and Potentially amphiphilic.
Magainin and Magainin 2
Magainin and Magainin 2 were discovered together, and posses very similar sequences, of 23 Amino Acid long.
Magainin: Gly-Ile-Gly-Lys-Phe-Leu-His-Ser-Ala-Gly-Lys-Phe-Gly-Lys-Ala-Phe-Val-Gly-Glu-Ile-Met-Lys-Ser
magainin 2: Gly-Ile-Gly-Lys-Phe-Leu-His-Ser-Ala-Lys-Lys-Phe-Gly-Lys-Ala-Phe-Val-Gly-Glu-Ile-Met-Asn-Ser
When the only difference is the 22th amino acid, Lys for Magainin and Here we will debate about Magainin 2 properties.
Structural highlights and antimicrobial mechanism
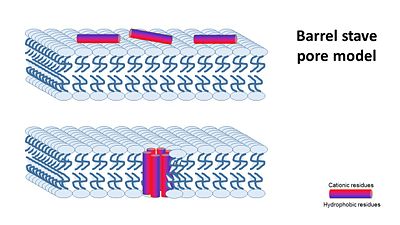
In general, amphipathic helical peptides that disrupt the ionic gradient of cells are thought to do so in various suggested mechanisms. One of the models is called the Barrel stave pore model. In this suggested mechanism peptides bind to the cell membrane, and in the seconed step the form ion channels assembled from 4–6 peptide molecules in the bacterial membrane. This results in the insides of the cell leaking outside, causing cell death.
(Image is according to Wimley, 2010.)
It was thought that this mechanism is also acountable for Magainin 2, But earlier solid-state NMR results show that its helix axis lies in the plane of phospholipid bilayers, suggesting that magainin’s mechanism for disrupting the ionic gradient may be fundamentally different. Therefore it's mechanism is still unclear, but either way it seems like Magainin 2 binds to the bacterial membrane to cause it's antibacterial effect.
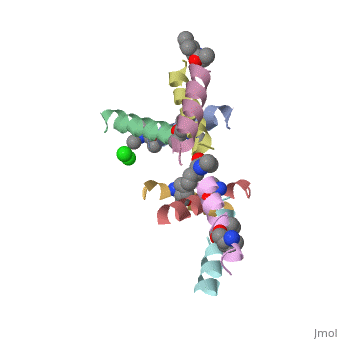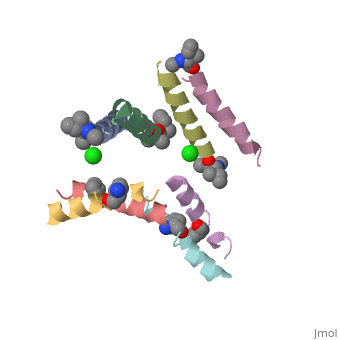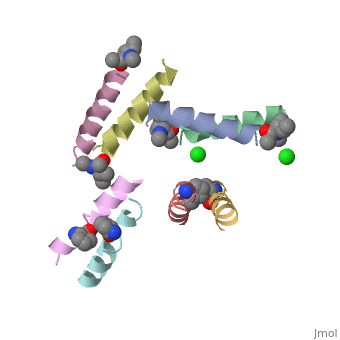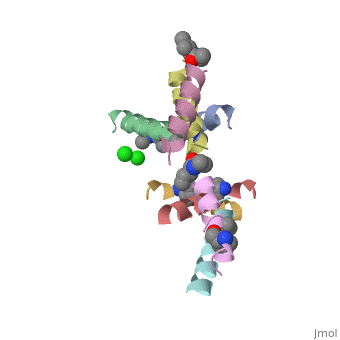
If we look at the Magainin 2 structure we can see how it allows Magainin 2 to bind to membranes:
Magainin 2, As typical to all AMPs, Is rich with that allow it to interact with Bacterial membranes, that are negatively charged in physiological pH. It is also rich with that allow it to interact with the membrane's phospholipids, and form a pore in the cell membrane.
As we mentioned, Magainin 2 can bind to lipid membranes through electrostatic attraction between it's positively charged residues and the negatively charged lipid membranes. It was shown that when surface charge density of the membrane was decreased, higher concentrations of Magainin 2 were required to induce leakage of cell content (Yukihiro & Yamazaki, 2009). These results support the assumption that positive residues allow Magainin 2 to bind to bacterial membranes.
Magainin 2 secondary structure also supports this assumption: We can see that the residues are organised in it's alpha helix in a way that one side contains all hydrophobic residues (shown in green), and the other side contains all cationic residues (shown in purple). This arrangment of all positive residues in one side probably helps Magainin 2 to bind to the bacterial membrane and perform it's antimicrobial action.
Crystalization of Ala-Magainin
So far we had shown Magainin 2 structure based only on NMR findings, Because helical AMPs crystallography is limited, since it is hard to form crystals.
In the Hayouka et al., 2013, In order to perform crystallization of Magainin 2, changes in the sequence were maid, and Ala-Magainin 2 was contsructed. In Ala-magainin one Ser (S) and two Gly (G) residues have been changed to Ala (A) in order to increase helical propensity.
Magainin 2: Gly-Ile-Gly-Lys-Phe-Leu-His-Ser-Ala-Lys-Lys-Phe-Gly-Lys-Ala-Phe-Val-Gly-Glu-Ile-Met-Asn-Ser
Ala -magainin 2: Gly-Ile-Gly-Lys-Phe-Leu-His-Ala-Ala-Lys-Lys-Phe-Ala-Lys-Ala-Phe-Val-Ala-Glu-Ile-Met-Asn
These changes resulted in minor changes in the secondary structure. we can see here that were changed to ala in . We can see Ala-Magainin has a few more residues in a alpha helix structure.
To form crystalyztion of Ala-Magainin 2,
was created.
Whilst racemic crystallization was not successful for magainin 2, Racemic crystalization was successful for Ala-magainin. This allowed finding the peptide's structure based on crystallization, that is more accurate than NMR