Methionine synthase
From Proteopedia
Contents |
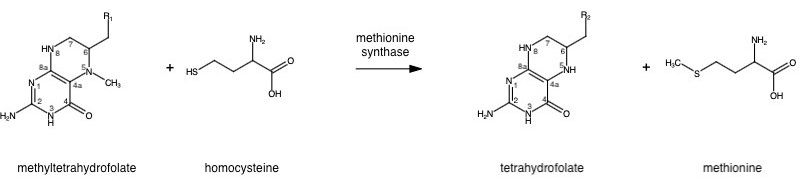
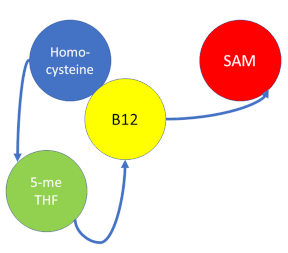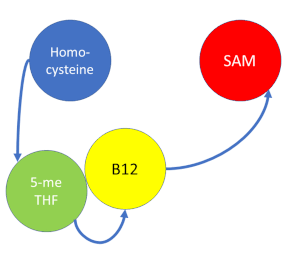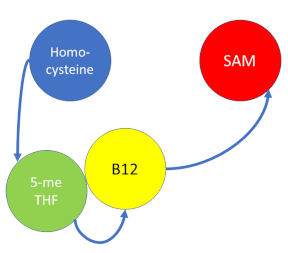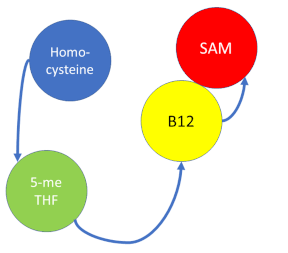
Methionine synthase (MS; EC: 2.1.1.13) or 5-methyltetrahydrofolate S-homocysteine methyltransferase is the enzyme in one-carbon metabolism linking the folate cycle to the methionine cycle. MS catalyzes the transfer of a methyl group from 5-methyltetrahydrofolate (5- me THF) to homocysteine, resulting in the formation of methionine and tetrahydrofolate (THF). Methionine is an essential amino acid required by our bodies for healthy cell and tissue growth. It is essential as it is not naturally derived in our bodies. As it is used as a methyl donor in the form of S-adenosylmethionine, the resulting homocysteine is recyled to form methionine again.
Function
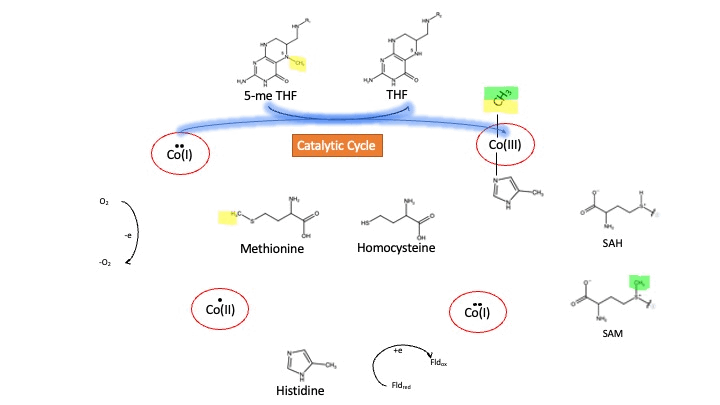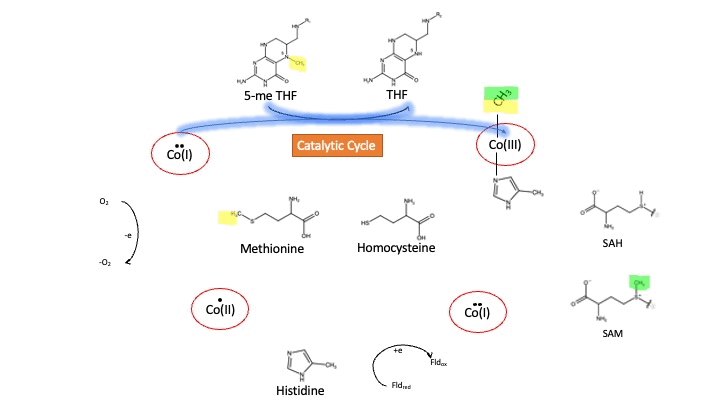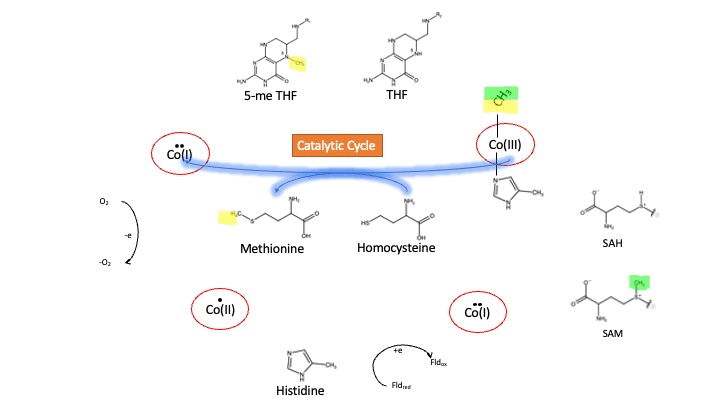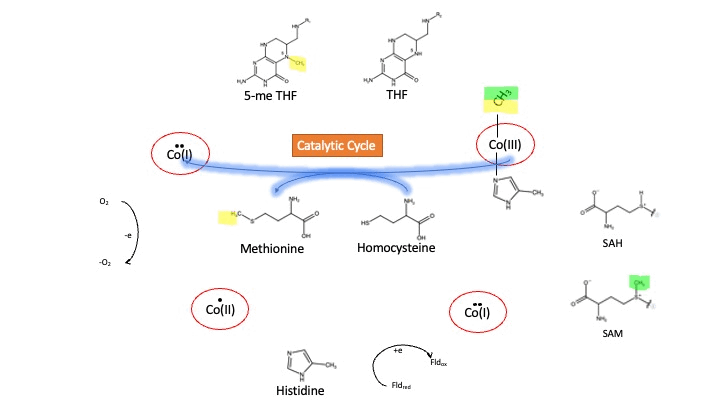
MS is a B12-dependent enzyme responsible for regenerating methionine from homocysteine. MS uses vitamin B12 Cobalamin as a cofactor. The change from homocysteine to methionine is an SN2 reaction where the methyl group on N-5 from 5-me THF is donated to Cob(I)alamin forming methylcobalamin (or Me-Cob(III)alamin). This is a complex reaction as THF, a product, is a poor leaving group and requires the "super nucleophile", Cob(I)alamin, to carry out the reaction[1][2] as a methyl carrier.
5-me THF is a product of methylenetetrahydrofolate reductase (MTHFR) from the folate cycle.
Oxidation States of Cobalamin
Cobalamin exists in three different oxidation states during the MS cycle.
Cob(I)alamin: Cobalt in the +1 oxidation state is nicknamed the "super nucleophile" as its high energy is required to carry out the complex SN2 reaction of breaking the bond between THF and the methyl group, in the catalytic cycle.
Co(III)alamin: Cobalt in +3 oxidation state occurs when His 759 displaces the dimethylbenzimidazole (DMB) ligand to allow for the methyl to be accepted by Cob(I)alamin, forming Me-Cob(III)alamin.
Cob(II)alamin: Cob(I)alamin is highly reactive towards oxygen so occasionally under aerobic conditions, Cob(I)alamin will undergo oxidation leading to an inactive Cob(II)alamin enzyme in the +2 oxidation state. This is regulated by reductive methylation by using Flavodoxin as an electron donor to reactivate Cob(I)alamin, and subsequently regenerates Me-Cob(III)alamin with a methyl being donated from SAM[3].
Relevance
MS is an important enzyme responsible for generating methionine, required by our bodies for healthy cell and tissue growth, and protein synthesis. Any MS and/or B12 deficiencies can result in diseases such as abnormal birth defects or anemia[2].
Structural highlights
Methionine synthase 3D structures
Methionine synthase 3D structures
| |||||||||||
Acknowledgements
Many thanks to Dr. Theis, Anna, Mike, and Shaylie for their enthusiasm and assistance on creation of 3D structural images of the B12 domain for MS.
Also thanks to Dr. Drennan for taking the time to review the page and providing helpful suggestions for improvement.
References
- ↑ Banerjee R, Ragsdale SW. The many faces of vitamin B12: catalysis by cobalamin-dependent enzymes. Annu Rev Biochem. 2003;72:209-47. doi: 10.1146/annurev.biochem.72.121801.161828. PMID:14527323 doi:http://dx.doi.org/10.1146/annurev.biochem.72.121801.161828
- ↑ 2.0 2.1 Kung Y, Ando N, Doukov TI, Blasiak LC, Bender G, Seravalli J, Ragsdale SW, Drennan CL. Visualizing molecular juggling within a B(12)-dependent methyltransferase complex. Nature. 2012 Mar 14. doi: 10.1038/nature10916. PMID:22419154 doi:10.1038/nature10916
- ↑ Bandarian V, Ludwig ML, Matthews RG. Factors modulating conformational equilibria in large modular proteins: a case study with cobalamin-dependent methionine synthase. Proc Natl Acad Sci U S A. 2003 Jul 8;100(14):8156-63. doi:, 10.1073/pnas.1133218100. Epub 2003 Jun 27. PMID:12832615 doi:http://dx.doi.org/10.1073/pnas.1133218100
- ↑ 4.0 4.1 Bandarian V, Pattridge KA, Lennon BW, Huddler DP, Matthews RG, Ludwig ML. Domain alternation switches B(12)-dependent methionine synthase to the activation conformation. Nat Struct Biol. 2002 Jan;9(1):53-6. PMID:11731805 doi:10.1038/nsb738
- ↑ Banerjee R, Ragsdale SW. The many faces of vitamin B12: catalysis by cobalamin-dependent enzymes. Annu Rev Biochem. 2003;72:209-47. doi: 10.1146/annurev.biochem.72.121801.161828. PMID:14527323 doi:http://dx.doi.org/10.1146/annurev.biochem.72.121801.161828
Proteopedia Page Contributors and Editors (what is this?)
Kia Yang, Karsten Theis, Michal Harel, Anna Postnikova, Michael O'Shaughnessy