We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Molecular Playground/Homo-dimeric RcdA
From Proteopedia
| |||||||||||
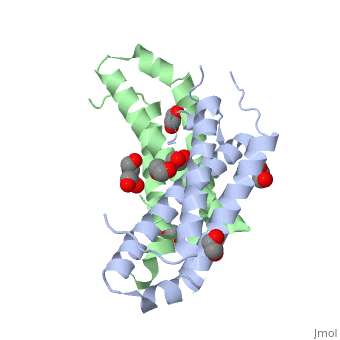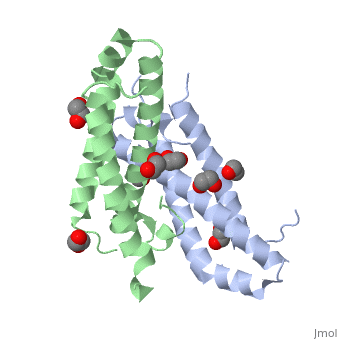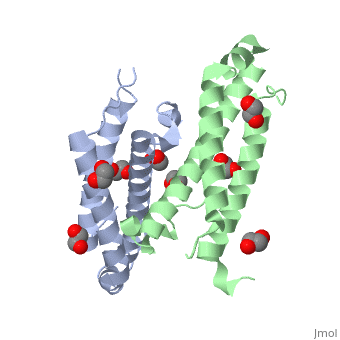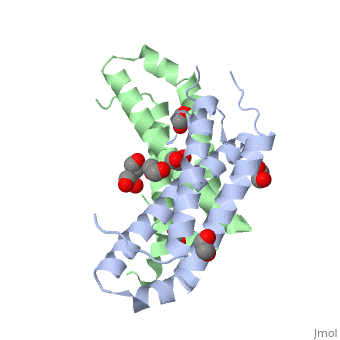
3D structures of RcdA
Updated on 23-October-2017
3ctw - RcdA - Caulobacter crescentus
References: 1. Patrick T. McGrath, Antonio A. Iniesta, Kathleen R. Ryan, Lucy Shapiro and Harley H. McAdams. " A Dynamically Localized Protease Complex and a Polar Specificity Factor Control a Cell Cycle Master Regulator". Cell, (2006) 124, 535–547. 2. James A. Taylor, Jeremy D. Wilbur, Stephen C. Smith and Kathleen R. Ryan. "Mutations that Alter RcdA Surface Residues Decouple Protein Localization and CtrA Proteolysis in Caulobacter crescentus". J. Mol. Biol. (2009) 394,46–60.
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Kamal K. Joshi, Alexander Berchansky, Joel L. Sussman