Function
Complex I is the first enzyme of the electron transport chain. This enzyme is one of the three large chains of protein complexes that electrons are transferred through. For every molecule of NADH that is oxidized, this complex moves four protons across the inner membrane and into the cytoplasm. This helps build the electrochemical potential used to produce ATP. The reaction of this complex is NADH + Q + 5H+(matrix) → NAD+ + QH2 + 4H+(cytoplasm)
Mechanism
NADH initially binds to Complex I and transfers its two electrons to the flavin mononucleotide (FMN) prosthetic group, reducing its form to FMNH2. These electrons flow through a series of Fe-S centers and then to coenzyme Q. This flow leads to the pumping of four hydrogen ions out of the matrix of the mitochondria. Q, upon accepting the two electrons, takes up two protons from the matrix and is reduced to QH2. All redox reactions take place in the extramembraneous part (hydrophillic) of NADH-Q oxidoreductase.
Structure
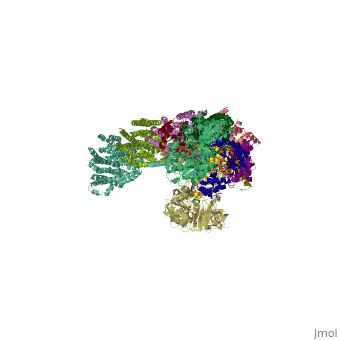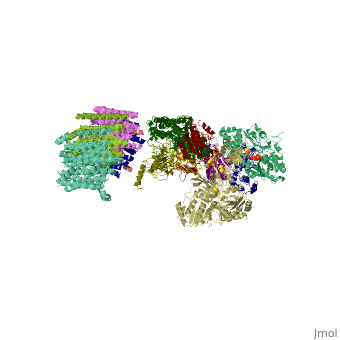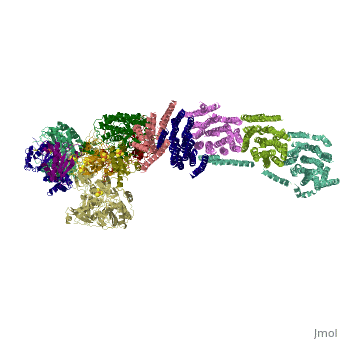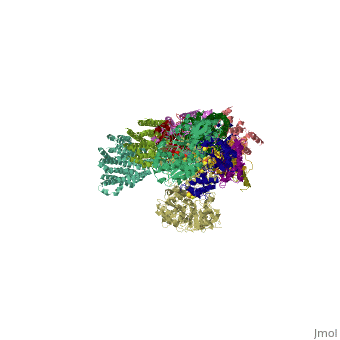
NADH-Q oxidoreductase consists of approximately 46 polypeptide chains (>900kd). The genes encoding this proton pump resides in both the mitochondria and the nucleus. This enzyme is L-shaped, with a horizontal arm lying the membrane and a vertical arm that projects into the matrix.
Disease
Structural highlights
This is a sample scene created with SAT to by Group, and another to make of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes.