Neurotensin Receptor (NTSR1)
Introduction
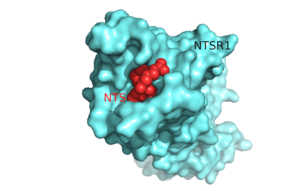
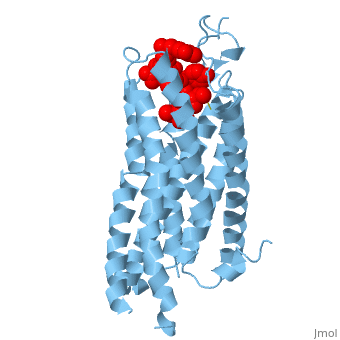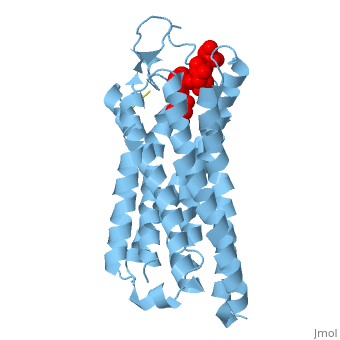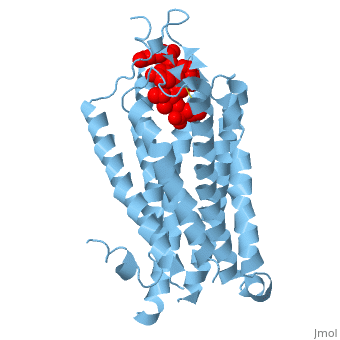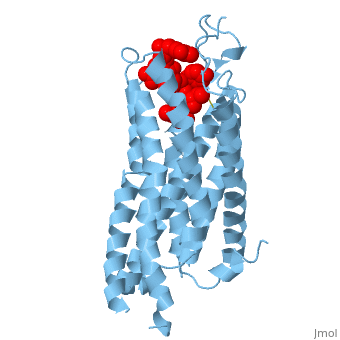
Figure 1.Top view of NTSR1 protein (blue) interacting with its ligand, NTS(red).
The neurotensin receptor (NTSR1) belongs to the superfamily of proteins known as
G protein-coupled receptors (GPCRs). Currently around 800 G protein-coupled receptors have been identified and are hypothesized to be responsible for roughly 80% of
signal transduction.
[1] GPCRs are involved in a vast array of physiological processes within the body that range from interactions with
dopamine to effects on secretion of bile in the intestines.
[2] [3] Due to the vast array of functions that these proteins serve and their high abundance within the body, these proteins have become major drug targets.
[4]
The ligand for NTSR1 is the 13 amino acid peptide, neurotensin (NTS)[5], and the majority of the effects of NTS are mediated through NTSR1[5]. NTS has a variety of biological activities including a role in the leptin signaling pathways [6], tumor growth [7], and dopamine regulation [8]. NTSR1 was crystallized bound with a C-terminal portion of its tridecapeptide ligand, . The shortened ligand was used because of oits higher potency and efficacy than its full-length counterpart[5].
A critical topic in the understanding of GPCRs is the transition from the inactive to active state. This transition is responsible for the transduction of a signal from the extracellular to the intracellular space. The transition occurs when a ligand, NTS in the case of NTSR1, binds to the receptor causing a conformational change that leads to the activation of the intracellular G protein. Currently, only the structure of the active form of NTSR1 is known, making the transition between the active and inactive states difficult to study.[5]
See also Transmembrane (cell surface) receptors
Neurotensin
Neurotensin (NTS) is a 13-amino acid peptide originally isolated from the bovine hypothalamus. [9] NTS fulfills the roles of both a neurotransmitter and a neuromodulator in the nervous system and a hormone in the periphery nervous system. NTS is a neuromodulator of dopamine transmission and of anterior pituitary hormone secretion. [10] In the periphery of the digestive tract and cardiovascular system, NTS is a paracrine and endocrine modulator.[11] Finally, NTS serves as a growth factor for many normal and cancerous cell types. [12] Only the C-terminal tail of NTS, amino acids 8-13, were resolved in the (PDB code:4GRV).
Structure
Like other G protein-coupled receptors, NTSR1 is composed of 3 distinct regions. An where neurotensin binds and causes a conformational change of the protein. A region containing (PDB code:4GRV) that transduce the signal from the extracellular side of the cell membrane to the intracellular side. Lastly, an intracellular region that when activated by a conformational change in the protein activates a G-protein associated with this receptor.
The in NTSR1 is located at the top of the protein (Figure 1). NTSR1 also contains an allosteric , which is located directly beneath the ligand binding pocket and the two pockets, which are separated by the residue [13]. NTSR1 has been mutated to exist in both and states.
Neurotensin Binding Pocket
On the extracellular side of the protein is the
. [5]Binding of NTS to NTSR1 is enriched by between the positive NTS arginine side chains and the negative pocket. In addition, the C-terminus of NTS forms a (PDB code:4GRV) with Arg328 of NTSR1. Three hydrogen bonds are made between the side chains of NTS and the receptor while most of the interactions are a result of Van der Waals interactions. The binding pocket is partially capped by a at the proximal end of the receptor's N-terminus.[5]
The binding of NTS to NTSR1 is also driven by hydrophobic stacking. One key residue in this pocket is a Phenylalanine at position 358, which takes part in a network of hydrophobic stacking interactions[13]. These interactions stabilize the Trp321 and Tyr324 residues allowing Tyr324 to interact with the C-terminal via Van der Waals interactions.[5][13] Without the hydrophobic stacking interactions that are facilitated by the Phe358, this binding interaction would be destabilized. Trp321 also participates in these stacking interactions and serves as the boundary between the ligand binding pocket and the Na+ binding pocket.[13] Another major player in the transduction of the extracellular signal to the intracellular G-protein is the (PDB code:4GRV)that links the bound hormone with the hydrophobic core of the neurotensin receptor. The carboxylate of Leu13 of NTS forms a hydrogen bond network with Arg327, Arg328, and Tyr324. The Tyr324, in turn, is brought into an orientation to make the formation of a (PDB Code:4XEE) network between Phe358, Trp321, Ala157, and Phe317 possible.[5] The effects that this network has on the activation of the intracellular G-protein was examined by the mutagenesis of amino acids that disrupted the formation of this network. Mutagenesis of , , , , , and showed that when this interaction was disrupted, the receptor no longer activated the intracellular G-protein.[5] This discovery lead to the conclusion that the conformational changes caused by this stacking allows for the signal to be moved from the extracellular binding site through the transmembrane helices of the receptor to the intracellular region activating the G-protein.
Na+ Binding Pocket
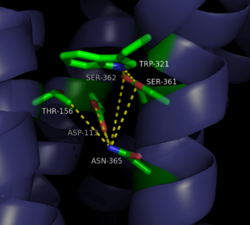
Figure 2: Closed form of sodium binding pocket that caps the entrance of sodium into the top of the binding pocket. (PDB Code:
4XEE)
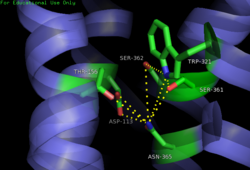
Figure 3: Open form of sodium binding pocket that does not cap the entrance of sodium into the top of the binding pocket. (PDB code:
4GRV)
Conserved across all class A GPCRs, a (PDB code:
4GRV) is seen in the middle of TM2 helix of NTSR1. The bound sodium ion is coordinated with a highly conserved Asp113 and four other oxygen contacts from a combination of water molecules. For G-protein activation, a hydrogen bond coordination with Thr156, Ser362, and Asn365 of the NPxxY
motif must occur. Trp321 helps to maintain the active conformation of the receptor by occluding the top of the binding pocket using
Van der Waals interactions (Figure 2). This occlusion stops sodium ions from entering the top of the binding pocket and helps NTSR1 remain in its active conformation. The conformation of the binding pocket where Trp321 does not occlude the top can be seen when mutations to A86L, G215A, and V360A are present (Figure 3). This form of the receptor allows more sodium into the binding pocket and stabilizes the inactive form of the receptor.
[14] , which is positioned at the bottom of the , sets the top of the . The Na
+ ion binding pocket acts as a negative allosteric site for G protein activity.
[13] When Na
+ enters the Na
+ ion binding pocket, it coordinates with Asp95, Gln131, Ser135, and Asp113, decreasing the signaling activity of NTSR1
[13]. When NTSR1 is in its active state, the Na
+ ion binding pocket is collapsed. This prevents the regulation of protein activity through a Na
+ ion, as the Na
+ ion is unable to coordinate via a salt bridge to Asp113 (Figure 3). The side chain atoms of Asp113 form a
hydrogen bond network with Thr156, Ser361, Ser362, and Gln365 instead, which prevents the coordination of a Na
+ ion
[13] (Figure 3).
Activation of NTSR1
Since wild type NTSR1 was unstable in detergent solution, six residues in the protein were mutated for stabilization.[5] [13]
Active-Like State
The six amino acid mutations for thermostabilization [5] were , , , , , and . This protein was found to have NTS affinity similar to that of wild tpye NTSR1, and was named . However, NTSR1-GW5 did not have G-protein activity.[5]
Active State
After determining that was only active-like, NTSR1 was recrystallized in a more fully active state.[13] By reverting back three of the original six mutations, NTSR1 regained near wild-type G-protein activity.[13] The three reversions were Asp166, Leu310, and Phe358, and this protein was named . The revival of activity in NTSR1 indicated that the reverted amino acid residues (Asp166, Leu310, and Phe358) play significant roles in G-protein activity.[13]
Leu310
is crucial for interactions with the G alpha subunit by positioning Arg167 in the conserved [13]. When Leu310 was substituted with alanine, Arg167 was able to form a stabilizing hydrogen bonding network with Asn257, Ser164 and Gly306, which oriented Arg167 in a position that was unfavorable for contacting the G alpha subunit. When residue 310 was converted back to leucine, this hydrogen bonding network was sterically unfavorable and Arg167 interacted with the G alpha subunit[13] leading to the transduction of several different signals involved in dopamine regulation[8], leptin signaling[6], and tumor growth[7].
Phe358
When this residue was mutated to an alanine [13] the of the ligand binding pocket were interrupted,resulting in a lack of G-Protein activity in NTSR1.[13].This supported the role of Phe358 in the hydrophobic binding pocket section.
Glu166
Although the role of in G-protein activity is not quite as clear as it is for or , substituting this residue for an alanine significantly reduced catalytic G-protein activity [13]. Glu166 is part of a that is highly conserved in class A GPCRs and includes Arg167 and Tyr168. Glu166 was hypothesized to interact with Val102, Thr101,and His105 to stabilize the G protein. An important connection between the D/ERY motif and intracellular loop 2 via Met181, has also been hypothesized.[13].
Biological and Clinical Relevance
Leptin Research
NTSR1 deficient mice were not able to receive a satiety signal from Leptin[6]. The mice continued to eat when food was present, leading to significant weight gain. With an NTSR1 deficiency, NTS does not bind efficiently to NTSR1, and the leptin signaling pathway is interrupted [6].
Cancer Studies
Some tumor cells can secrete and express NTS and NTS receptors, suggesting that NTS autocrine, endocrine and paracrine regulation are possible. This leads to aggressive growth and tumor development. Injecting animals with NTS increased tumor growth and size, while injecting them with NTS antagonist had the opposite effect.[7]
Dopamine Regulation
The dopamine hypothesis states that hyperdopamine levels may lead to schizophrenic symptoms. NTSR1 causes a blockade which inhibits firing in dopaminergic cells suggesting that NTSR1 could be used in schizophrenia treatment. However, this led to extreme secondary effects and was discontinued. Despite this, research on NTSR1 as a treatment for schizophrenia persists[8].
Clinical Relevance
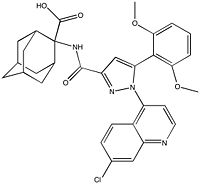
Figure 4: Meclinerant: An inhibitor of NTSR1 found to enhance selectivity of radiotherapy in cancer treatment.
NTSR1 is commonly expressed in various invasive
cancer cell lines making it a promising cancer drug target. It is prevalent in
colon cancer adenocarcinoma, but is not found in adult colon cell types.
[15] NTSR1 is also found in aggressive
prostate cancer cells, but not
epithelial prostate cells. In prostate cancer cells, binding of NTS results in
mitogen-activated protein kinase (PKB),
phosphoinositide-3 kinase (PI-3K),
epidermal growth factor receptor (EGFR),
SRC, and
STAT5 phosphorylation.
[15] These all result in increased DNA synthesis,
cell proliferation, and survival. Inhibition of NTSR1 and its downstream signaling represents a target for
radiotherapy, which uses radiation to target malignant cells. NTSR1 can be inhibited by agonist
meclinertant which inhibits proliferation and prosurvival of cancer cells. Combination treatment of radiation and meclinerant provides selective treatment of cancer cells over normal cells, indicating the need for clinical trials of this approach.
[16]
Student Contributors
Allie Cotter
Danny Cotter
Andrew Koelper
Brent Waibel
3D structures of neurotensin receptor