Introduction
The NOMPC (No mechanoreceptor potential C) is a mechanosensitive ion channel essential for hearing, touch and locomotion (proprioception) in Drosophila melanogaster. It responds directly to mechanical stimuli such as pressure and stretch, converting physical forces into electrical signals. NOMPC is classified as a transient receptor potential (TRP) channel, a superfamily of membrane bound proteins involved with stimulus detection and sensory transduction in various animal cells. When first discovered, NOMPC introduced a novel class of receptors, the TRPN. Although it was first described in Drosophila melanogaster [1], homologs of NOMPC were found in other animals, including the nematode worm Caenorhabditis elegans and the zebrafish.
Structure and mechanism of action
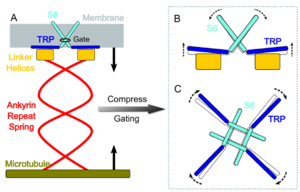
Figure 1. Schematic representation of NOMPC mechanism of action. The ankyrin repeat domains form a spring-like helical structure that links to microtubules. Cytoskeleton displacement generated by a sound or pushing forces will subsequently generate tension and torque on the linker helix domain. As a result, the transmembrane S6 helices (the sixth alpha-helix of the transmembrane domain) of the tetramer are rearranged. This leads to the pore formation. Subsequent calcium-ion influx causes the membrane to depolarize and trigger a potential of action. Note the participation of the TRP domain in S6 movement and pore formation. (Source: Wang et al. Elife. 2021;10:e58388. doi:10.7554/eLife.58388.)
NOMPC structure was first resolved in 2017 using Cryo-EM technique [2], with a resolution of 3.6 Å. At this resolution, models can distinguish between atoms. This level of detail is essential for understanding the mechanism of action of this protein.
NOMPC is a homotetramer , meaning its biologically functional arrangement is formed by the non-covalent interaction between four equal subunits, i.e. monomers. The monomers interact with each other through domain-swap interactions, where each monomer contributes part of its structure to stabilize its neighbors [3], leading to a stable, ring-like assembly. Effectively, the monomers interlock.
By resolving its structure, researchers highlighted the presence of 29 ankyrin repeats per monomer, at the N-terminus. This is the largest number among TRP channels. An ankyrin repeat is a motif of about 33 amino acids repeated in tandem (i.e., one behind the other). Each repeat adopts a helix-turn-helix conformation. Ankyrin repeats are known to mediate protein-protein interactions [4][5].
As other TRP channels, NOMPC has a characterized by a series of six alpha-helices (S1–S6). Additional connect the ankyrin repeat domain and the transmembrane domain. Following the C-terminus portion, there is an intracellular . The rest of the C-terminus is mostly unstructured.
Although C-terminal, the TRP domain is “sandwiched” between the linker helices and the transmembrane portion, when looking at NOMPC tertiary structure (see Figure 2 and the 3D visualization). This positioning is important since the TRP domain movement regulates the pore formation (see Figure 1) [6].
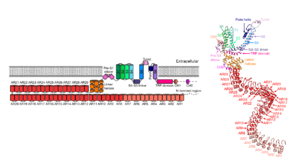
Figure 2. Monomeric representation of NOMPC. Left: two-dimensional schematic. Right: tertiary structure of NOMPC modelled with Cryo-EM data. (Source: Jin et al. Nature. 2017;547(7661):118-122. doi:10.1038/nature22981)
NOMPC gating is thought to be triggered by tethering of its ankyrin repeat domain to microtubules of the cytoskeleton. Structural modelling of this protein suggests that the ankyrin repeat domain in each monomer forms a helical spring. This structure suggests an interaction between the NOMPC and the cytoskeleton, and could suggest the opening of the ion channel in response to cytoskeleton displacement.
A graphical representation of this proposed mechanism of action is shown in Figure 1.
However, a recent study [7] challenged this gating mechanism. Their results suggest that it is the linker helices (LH) domain, not the ankyrin repeat (AR) domain, that mediates channel gating. They have shown that LH is ten times more elastic than AR domain. And also, they have shown that while duplication of AR domain does not significantly alter NOMPC gating, duplication of LH domain reduces mechanosensitivity. In conclusion, LH domain is responsible for over 90% of gating-spring compliance, while AR domain is mostly functional for tethering the channel to the cytoskeleton.
Biological functions
NOMPC is a transient receptor potential mechano-electrical transduction (MET) channel from the fruit fly, Drosophila melanogaster. It is implicated in touch sensation, proprioception and hearing.
The sensation of touch, gravity and sound allows animals to perceive its environment and to navigate through it (literally, since these sensations are required for coordinated body movement). It all starts with the transduction of mechanical stimuli into electrical signals [7]. Mechanosensory cells mediate this mechano-electrical transduction (MET) through MET channels — ion channels gated directly by mechanical stimuli. When this gating mechanism was uncovered in the 1980’s (in hair cells of the inner ear), it was observed that it requires an elastic element that couples cell deformation to pore formation.
Locomotion and proprioception
NOMPC is essential for proper locomotion and larval crawling [8]. It mediates proprioception, which is the sensory input and feedback by which animals control body parts for balance and movement. Its proper function influences the pace of larval crawling. Mutations in NOMPC can result in severely uncoordinated adult flies and dramatically reduced crawling speed in larvae. NOMPC is required for activating specific sensory neurons during peristaltic muscle contractions in larvae, which are critical for locomotion. Its function in sensory neurons is also essential for adult locomotion [8].
Touch sensation
NOMPC functions as a mechanotransduction channel for gentle-touch sensation [9]. Loss-of-function mutations in NOMPC abolish mechanoreceptor potentials in fly bristles, and a missense mutation in the channel alters the adaptation of these potentials. Ectopic expression (i.e. gene expression in a cell, tissue, or developmental stage in which the gene is not usually expressed) of NOMPC can even confer touch sensitivity to normally touch-insensitive neurons [9].
Hearing
NOMPC is essential for hearing in Drosophila [2][8] and is particularly important for the function of sound receptors in the fly's antennal hearing organ [7][10]. It is required for the nonlinear mechanical amplification of antennal vibrations, a process that enhances the mechanical sensitivity to faint sounds. Loss of NOMPC function reduces auditory sensitivity and the amplitude of sound-evoked nerve responses [10].
NOMPC homologs
Through genomic analysis, homologs of NOMPC have been reported in most animals, including all Bilateria except for amniotes [11]. These NOMPC homologs are members of TRPN family and show remarkably conserved domain arrangement (e.g. the presence of 28 or 29 ankyrin motifs is ubiquitous).
Below there is a list of NOMPC homologs found in other animals. The links redirect to NCBI gene entry.
- Caenorhabditis elegans (Nematode worm): trp-4
- Xenopus laevis (African clawed frog): nompc
- Danio rerio (Zebrafish): trpn1


