Sandbox 213
From Proteopedia
|
Calmodulin (CaM) for Calcium-Modulated protein is an important protein that intervenes in a wide range of activities[1].
CaM is expressed in many cell types and can have different subcellular locations, including the cytoplasm, within organelles, or associated with the plasma or organelle membranes. Indeed, it is a small (16.7 kDa = 148 aa) and highly conserved protein that is necessary in all eukaryotic cells because it represents an essential calcium sensor with troponin C its isoform.
Calcium is an important mineral salt which represents 2% of body weight and 99% in teeth and bones. Calcium levels are finely regulated in the organism because it ediates and controls many biochemical processes, inflammation, metabolism, apoptosis, smooth muscle contraction, intracellular movement, short-term and long-term memory, and the immune response. Calcium ions play a major role in the metabolism and physiology of eukaryotes cells, that's why calmodulin is important for the organism.
Contents |
Biological function
Calmodulin is a receptor protein owing a wide ranging effects. About 30 enzymes and structural proteins have been identified that bind calmodulin with high affinity. The Ca2+- calmodulin complex can regulate the intracellular concentration of some second messengers such as cyclic AMP and inositoltriphosphate (IP3)
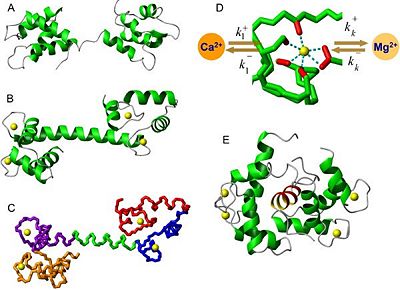
Structure
| |||||||||||
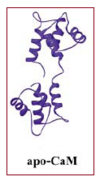
- Three-dimensional structure of apocalmodulin
In the absence of bound Ca2+, the helices of calmodulin pack so that their hydrophobic side chains are not exposed. In this form it is unable to interact with its targets.The C-terminal lobe of each CaM adopts a semi-open conformation that grips the first part of the IQ motif (IQxxxR), whereas the N-terminal lobe adopts a closed conformation that interacts more weakly with the second part of the motif (GxxxR). [4].
Apocalmodulin (ApoCaM) binds to other proteins and has other specific effects. It is essential role for life. The ApoCaM-binding proteins are a diverse group of at least 15 proteins including enzymes, actin-binding proteins, as well as cytoskeletal and other membrane proteins, including receptors and ion channels. [1].
- Ca2+-bound calmodulin
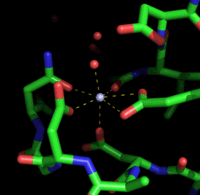
Calmodulin uses a linear sequence of 12 amino acids to bind Ca2+. Binding of Ca2+ to the four sites induces a large conformational change causing the terminal helices to expose hydrophobic surfaces and also a long central α-helical segment. Ca2+-bound calmodulin binds to its targets with high affinity (KD≈10−9 mol.L−1)[5].
- Calmodulin bound to a target peptide
|
To form the bound state, the central residues of the link region unwind form their α-helical arrangement to form a hinge that allows the molecule to bend and wrap itself around the target. The N-terminal and C-terminal regions approach each other and by their hydrophobic surfaces bind to it, rather like two hands holding a rope. This encourages the target sequence to adopt an α-helical arrangement so that it occupies the center of a hydrophobic tunnel. The consequence of this interaction is a conformational change in the target, a state that persists only as long as the Ca2+ concentration remains high [5].
When the Ca2+ concentration falls, calcium dissociates and calmodulin is quickly released, inactivating the target. However, at least one important target protein is an exception to this rule. This is CaM-kinase II which can retain its active state after it has been activated by calmodulin.
CaMII kinase
Calmodulin plays an important role through kinase enzymes such as calcium/calmodulin-dependent kinase II (CaMKII) that is a multifunctional serine/threonine kinase found in many tissues. Activation of CaMKII contributes to synaptic plasticity and regulation of excitory synaptic transmission. The regulatory domain of CaMKII contains an autophosphorylation site, which is essential for its calcium-dependent activation [6].
CaM kinases have a catalytic N-terminal domain, a regulator domain and a domain of association. Many CaM form an holoenzyme with a dodecameric structure(the catalytics domains are found one's way out side) that permit to phosphorylate the residues between the sub-unit. Without Ca2+/calmodulin, the catalytic domain is self-inhibited by the regulator domain, which contains one sequence of type pseudo-substrate. Many CaM kinases give an homo-oligomeres or hetero-oligomeres. When there is an activation by the Ca2+/calmodulin complex, the actif CaM kinases are autophosphorylated one by one at the level of the threonine 286 residues.
Family members
- Calmodulin 1 (CALM1)
Synonyms: CALM, CAM, CAM1
- Calmodulin 2 (CALM2)
Synonyms: CAM2, CAMB
- Calmodulin 3 (CALM3)
Synonyms: CALML2, CAM3, CAMC, CAMIII
- Calmodulin-like 1 (CALML1)
- Calmodulin-like 3 (CALML3)
- Calmodulin-like 4 (CALML4)
- Calmodulin-like 5 (CALML5)
- Calmodulin-like 6 (CALML6)
Mutation of Calmodulin gene and consequences
Calmodulin is an essential protein that is encoded in vertebrates by tree different genes (CaMI, CaMII and CaMIII) whereas it is encoded by a single gene in yeast and fungi (CMDI). Consequently the disruption of CMDI in yeast and fungi is lethal. However studies on Saccaromyces cerevisae had shown that vertebrate calmodulin can rescue yeast cells whose calmodulin has been deleted. That experience indicates that vertebrate calmodulin can functionally substitute for yeast calmodulin because Saccaromyces cerevisae calmodulin amino acids sequence is 60% identical to vertebrate Calmodulin.
Calmodulin in laboratories
Because of widespread interest in calmodulin research many useful reagents are now available commercially. Purified calmodulin, anti-calmodulin, calmodulin-sepharose or -agarose and several calmodulin deficient target enzymes are available from major biochemical suppliers. For laboratories that plan extensive calmodulin research it may be more economically feasible to produce at least some of their reagents by various protocols such as calmodulin bioassay or calmodulin radioimmunoassay.
External Resources
- Protein Data Bank file on 2CLP
- Biochimie générale 10ième édition. Jacques-Henri Weil 2005
- Signal Transduction Gomperts.Kramer.Tatham Seconde edition
References
- Valeyev NV, Bates DG, Heslop-Harrison P, Postlethwaite I, Kotov NV. Elucidating the mechanisms of cooperative calcium-calmodulin interactions: a structural systems biology approach. BMC Syst Biol. 2008 Jun 2;2:48. PMID:18518982 doi:10.1186/1752-0509-2-48
- Halling DB, Georgiou DK, Black DJ, Yang G, Fallon JL, Quiocho FA, Pedersen SE, Hamilton SL. Determinants in CaV1 channels that regulate the Ca2+ sensitivity of bound calmodulin. J Biol Chem. 2009 Jul 24;284(30):20041-51. Epub 2009 May 27. PMID:19473981 doi:10.1074/jbc.M109.013326
- Houdusse A, Gaucher JF, Krementsova E, Mui S, Trybus KM, Cohen C. Crystal structure of apo-calmodulin bound to the first two IQ motifs of myosin V reveals essential recognition features. Proc Natl Acad Sci U S A. 2006 Dec 19;103(51):19326-31. Epub 2006 Dec 6. PMID:17151196
- Colbran RJ, Brown AM. Calcium/calmodulin-dependent protein kinase II and synaptic plasticity. Curr Opin Neurobiol. 2004 Jun;14(3):318-27. PMID:15194112 doi:10.1016/j.conb.2004.05.008
- Fallon JL, Quiocho FA. A closed compact structure of native Ca(2+)-calmodulin. Structure. 2003 Oct;11(10):1303-7. PMID:14527397
- Evans TI, Shea MA. Energetics of calmodulin domain interactions with the calmodulin binding domain of CaMKII. Proteins. 2009 Jul;76(1):47-61. PMID:19089983 doi:10.1002/prot.22317
Proteopedia page contributors and editor
Tonazzini Saphia, Planchenault Charlène