Calmodulin
Calmodulin (CaM), short for calcium modulated protein, is a small calcium binding protein with a three dimensional structure that allows calcium ions to come into the cells. Calcium is an essential mineral that is needed in the human diet for proper functioning of neurons to forming strong bones and can also act as a second messenger for enzymes and proteins. Calmodulin is known to be involved in various Ca2+ - dependent signal transduction pathways, the protein act as a Ca2+ detector, and the protein is involved with regulating protein-kinases [1].
Its' importance can be exemplified by the fact that the protein is known to be highly conserved in eukaryotes. Highly conserved structures that do not undergo significant evolutionary changes imply that the structure is mandatory for cell or organism survival and that any mutations in the genetic sequence that codes for the protein would be deleterious. The function of calmodulin is typically studied using yeast as a model organism. This is done for a variety of reasons, including the fact that yeast has a fully annotated genome with human homologues for genes associated with their ion channels, yeast is fast growing and yeast are heat stable[2]. Due to its’ ability to be easily manipulated, yeast can continue to be used to gather more information on calmodulins’ structure and function.
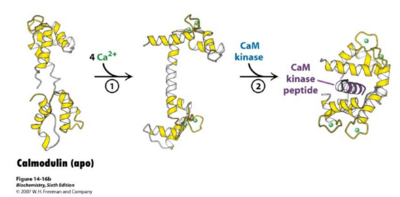
Figure 1: Calmodulin binds to 4 Calcium Ions and Undergoes Conformational Changes The figure highlights the structural importance of the flexibility of calmodulin in order to bind better to the 4 calcium ions at the ends and to undergo reactions with the CaM kinase peptides in order to begin a signaling transduction pathway. Click on the thumbnail to enlarge figure.
[3] Calmodulin in the body
Calmodulin is located and used ubiquitously by cells, but is especially prevalent in brain and muscle tissue. It has also been found in human serum, breast milk, urine, and saliva[4]. Calmodulin in the cell is mainly localized within organelles and binds to Calcium. Calcium binding then promotes the phosphorylation of protein kinases and activation of other proteins to begin signal transduction for a variety of different pathways, mainly different forms of cell signaling. The phosphorylation of these protein-kinases occurs when Ca2+ reach about 1000 nM and initiates a rapid signaling pathway[5].
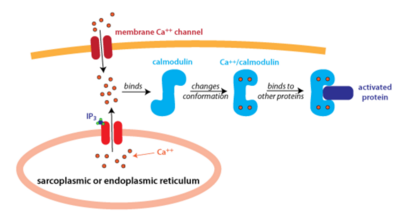
Figure 2: An Overview of Calmodulin Pathway Calmodulin binds to 4 Calcium Ions and Undergoes Conformational Changes
[6] Structural Highlights
Calmodulin has a molecular mass of 16 kilodaltons (kD) and it functions along with ryanodine receptor (RyR)[7]. CaM consists of 148 amino acid residues and is characterized by a helix-loop-helix binding motif, also known as the [8]. Calmodulin has one subunit with a distinct dumbbell shape in which a linker region joins two globular domains[9]. Calmodulin is known to undergo a conformational change upon binding with a calcium ion in which each lobe transitions from a closed conformation to an open conformation[10]. This protein has four major, high-affinity binding sites, as shown by figure 1. The calmodulin has been shown to be a series of hydrophobic amino acids (such as Trp or Leu), hydrophilic amino acids (such as Glu or Asp), and basic amino acids (such as Arg or Lys)[11]. Calmodulin typically wraps around its target, with the two globular domains gripping either side of it (Figure 1). NMR studies clearly show that the connector between the two calcium binding globular domains is flexible even when it is not bound to its target proteins. However, the full range of flexibility can be seen in calmodulin interactions with its target proteins (Figure 1).
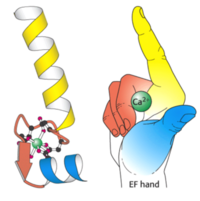
Figure 3: An illustration of the EF hand The yellow helix represents the ‘E’ portion and the blue helix represents the ‘F’ portion. The cavity inside the hand is where Ca2+ ions bind which induces the conformational changes in the loop region
[12]
Function
Each end of the of CaM binds to two Calcium ions, which allows CaM to bind to a total of four Calcium ions. The conformational changes which CaM undergoes allow it to be able to bind more specifically (Figure 1). Calmodulin elicits a pathway signal transduction by activating protein kinases which can then go on to phosphorylate other proteins, or other proteins can directly bind to Calmodulin[13]. This would require that the other proteins have a specific binding motif or substrate binding mechanism for Calmodulin (Figure 2). Because there are many different types of binding motifs used by other proteins to interact with Calmodulin, there are no conserved amino acid sequences for CaM binding. EF-hand motifs are a common type of calcium binding motif. These motifs can be characterized as having a helix-loop-helix pattern with 12 sequence residues. Aspartic acid, asparagine, glutamate, and serine are common residues found in this motif. By forming a loop, the motif allows calcium to bind more efficiently and securely (Figure 3). Once inside the binding site, calcium can then induce a conformational change.[14].
Some functions of calmodulin are associated with apoptosis, inflammation, metabolism, and smooth muscle contraction[15]. Based on a study done on Drosophila neuronal cells, calmodulin plays a role in the apoptotic pathways[16].




