Sandbox 645
From Proteopedia
HIV-1 Protease
Human Immunodeficiency Virus-1 Protease
IntroductionHIV -1 protease (HIV PR ) is a retroviral aspartyl protease that is derived from HIV-1, a lentivirus that is best characterized for its ability to lower host immunity by infecting CD4+ T lymphocytes, macrophages, and dendritic cells.[1] Aspartyl proteases are protease enzymes that utilize aspartate residue(s) for the catalysis of peptide substrates. Eukaryotic forms of these proteases include the , cathepsins and renins. While they have a two-domain structure, the retroviral aspartyl proteases are much smaller and homologous to a single domain of the eukaryotic aspartic proteases. HIV-1 protease is essential for the life cycle of HIV. The protease takes newly synthesized polyproteins and cleaves them by means of a hydrolysis reaction into the smaller mature protein components of the HIV virion.[2] These proteins are used to form the virion capsid that encases the viral genome. Effectively inhibiting HIV-1 protease will result in the inability for HIV to propagate as it will no longer have the ability to form complete virion units to infect new cells. The HIV-1 pol gene encodes for three enzymes; reverse transcriptase (RT), integrase (IN) and protease (PR). The HIV PR, together with single stranded RNA (ssRNA), reverse transcriptase, integrase, and other viral factors, is found inside the HIV-1 virion.[3]
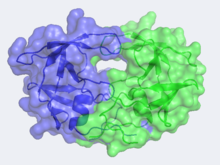
Structure & Functionof HIV-1 Protease In the mid 1980's, the structure of HIV-1 Protease was hypothesized by Lawrence Pearl and William Taylor to consist of a single domain of the eukaryotic aspartic protease and to function in dimeric form.[5] As NMR was not in wide use at this time, x-ray crystallography was implored but required a large quantity of large crystals. This was problematic as heavy atom derivatives had to be used to overcome the phase problems with this new structure and a considerable amount of protein was needed. Eventually these problems were overcome and the first images of HIV-1 protease where announced by the Merck group, who were most successful at large-scale protein purification and crystallization. Unlike most members of the aspartyl protease class, which generally exist as two domain monomers, HIV protease is a dimmer with two identical that are comprised of 99 amino acids. These two subunits come together to form a very narrow 7.46 angstrom diameter ; enclosing the active site in the middle. Instead of one flap (a long flexible β-hairpin loop from the N-terminal domain) closing over the active site like in pepsins, the homodimeric retroviral enzyme has two poorly ordered flaps.[6] The flaps contribute to the substrate binding site by containing two aspartyl residues on the hydrophobic interior side. When the active site is unligated, is hydrogen bonded to a water molecule at the turn of each flap. The polyprotein is capable of making it to the active site not by squeezing through the narrow tunnel but rather by having the flaps . The water molecules hydrogen bonded to the Ile50 seem to play a pivotal role in the opening of the flaps as well as increasing the substrate-enzyme binding affinity. Each subunit donates the catalytic triad, Asp-Thr-Gly, to the highly conserved active site, which also closely resembles that of monomeric proteases like pepsin. The residues are Asp25, Thr26 and Gly27 on each of the subunits. The active site is in the retroviral aspartyl protease just as in the eukaryotic aspartyl proteases. As will be seen in the mechanism, the two will hydrolytically cleave the polyprotein that comes into the active site.
MechanismThe mechanism most commonly accepted for the aspartyl proteases is a general acid-base mechanism involving the coordination of a water molecule between the two highly conserved aspartate residues which leads to hydrolysis. One of the aspartate residues induces activation of the water molecule by removing one of the protons. This allows the nucleophilic oxygen to attack the carbonyl carbon of the peptide and form a tetrahedral oxyanion intermediate. Rearrangement leads to the protonation of the scissile amide and thus cleavage. Applications & ResearchCurrently there is no cure for HIV. However, after some extensive research some drugs have been designed to allow the propagation of the HIV virions to be halted. These drugs are known as HIV-1 inhibitors. This image displays the protease bound to hydroxyethylamine. The ability to stop propagation is achieved by designing the drugs to mimic the transition state of the substrate and prevent the polypeptide from being hydrolyzed by the Asp residues. This is accomplished by having the peptide linkage of -NH-CO- changed to a hydroxyethylene group -CH2-CH(OH)- which the protease cannot cleave by hydrolysis. When the HIV infects an organism it attaches to the CD4 antigen located on the body's T cells and harnesses the T cell's machinery to replicate its own proteins and DNA. After successfully making more virions in the T cell, the virions break through the T cell membrane, causing the cell to die, and move on to infect other T cells in the body. This ultimately depletes the body's ability to fight infection as the T cells are pivotal cells in the body's immunity system.
Based on the crystal structure data and protein receptor ligand complexes studied, interatomic interactions that work on burying atoms and find the statistical preference for amino acid pairs. A free energy model of the receptor-ligand is formulated and helps in showing the interfacial interactions. The interaction strength of this model has a reliability of ±1.5 kcal/mol, which reveals the importance of atomic interaction to stabilize the receptor-ligand interface. The analysis of a binding motif of HIV-1 protease inhibitor complex shows the important contacts instead of the set of atoms. [7]
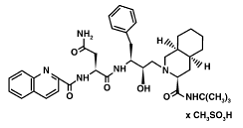
1) Saquinavir (Invirase) is known to be one of the first FDA approved protease inhibitor for HIV treatment. This usually occurs by HIV protease binding an active site , which will prevent polyproteins from also binding. Saquniavir's chemical structure has the ability to intermediate of the hydrolytic reaction to interact .
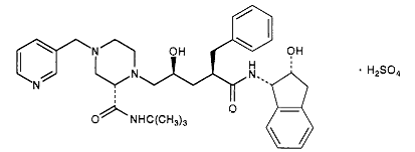
2) Indinavir (Crixivan) this protease inhibitor is an oral medication that blocks the protease activity and leads to defect the viruses. Once these viruses are unable to reach the body’s cells, it will decrease in number. This inhibitor doesn’t have the ability to prevent the HIV transmission among individuals, nor does it cure AIDS or HIV infection. (Crixivan)is approved by the FDA in March 1995. [9]
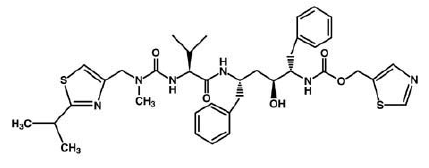
3) Ritonavir (Norvir) , has the same effect of indinavir on HIV protease. It was approved by the FDA in June 1999. This drug consists of capsules or tablets 100 mg; Solution: 80 mg/ml. It is recommended that adults consume 600 mg twice a day and then increase by 100 mg in every 2 days.
4) Nelfinavir (Viracept), Nelfinavir was approved by the FDA in March, 1997. (Viracept) has not been effectively evaluated in pregnant women. Viracept prevents T-cells that have been infected with HIV from producing new HIV. Usually given to patients through tablets. Each tablet contains inactive ingredients such as: calcium silicate, magnesium stearate, hypromellose, and triacetin.[11]
Future Research FocusThe main goals for future research in this field are: i) The design of better pharmacological agents while also avoiding severe side effects. ii) Achieving eradication of the virus and possibly a definitive cure for those infected. As has been mentioned previously, the pol gene of HIV-1 encodes for three enzymes; protease (PR), reverse transcriptase (RT) and integrase (IN). More than 10 drugs targeting two of these enzymes, PR and RT, have been approved by the FDA. At present, there are no clinically useful agents that inhibit the third enzyme, integrase. Researchers are trying to emphasize the proposed drug binding sites on IN in order to find successfully binding inhibitors.[12] This, along with continued research into inhibition design with HIV-1 Protease, will add to the foundation of research into defeating HIV.[13]
References
| ||||||||||||