This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Sandbox GGC15
From Proteopedia
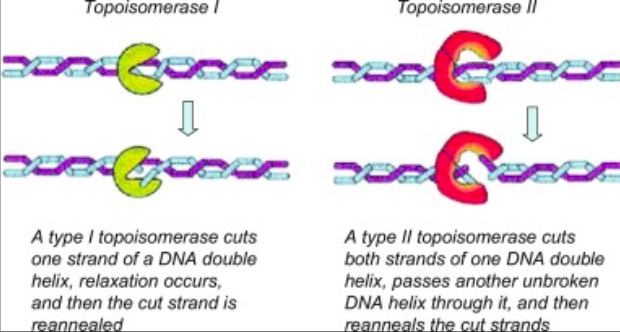
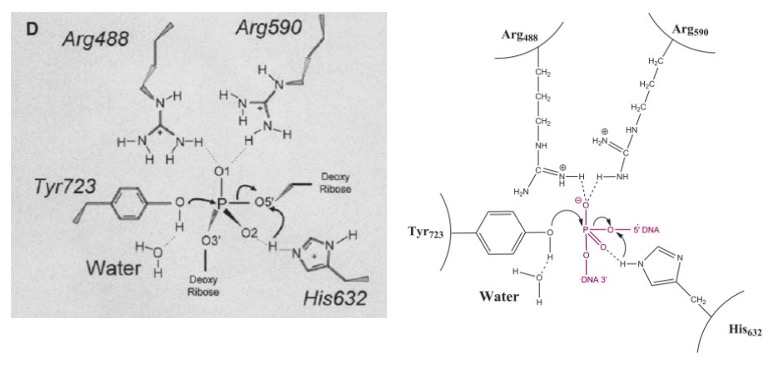
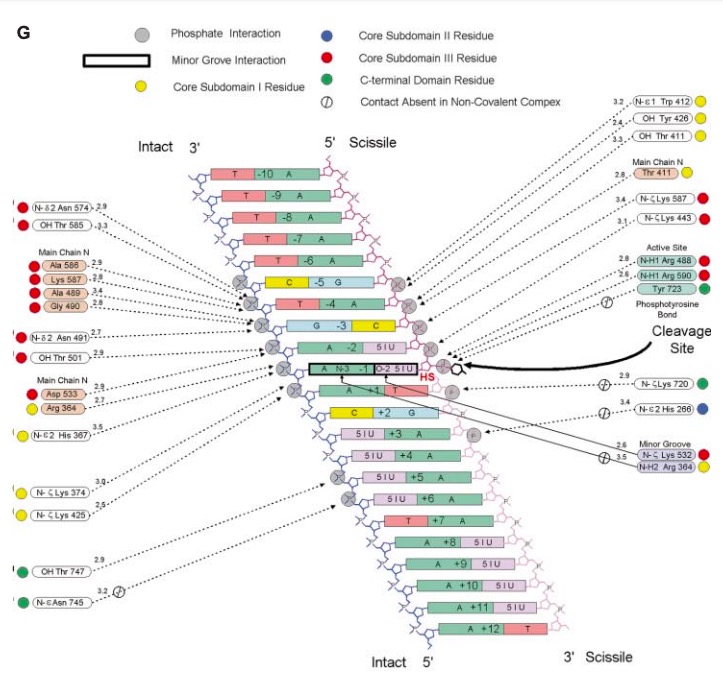
DNA TOPOISOMERASE I
| |||||||||||
References
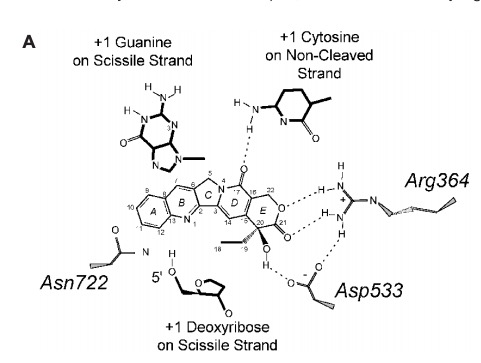
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 Staker BL, Hjerrild K, Feese MD, Behnke CA, Burgin AB Jr, Stewart L. The mechanism of topoisomerase I poisoning by a camptothecin analog. Proc Natl Acad Sci U S A. 2002 Nov 26;99(24):15387-92. Epub 2002 Nov 8. PMID:12426403 doi:10.1073/pnas.242259599
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 2.12 2.13 2.14 2.15 2.16 2.17 Redinbo MR, Stewart L, Kuhn P, Champoux JJ, Hol WG. Crystal structures of human topoisomerase I in covalent and noncovalent complexes with DNA. Science. 1998 Mar 6;279(5356):1504-13. PMID:9488644
- ↑ D'yakonov, V. A., Dzhemileva, L. U., & Dzhemilev, U. M. (2017). Advances in the Chemistry of Natural and Semisynthetic Topoisomerase I/II Inhibitors. Studies in Natural Products Chemistry, 21–86. https://doi.org/10.1016/b978-0-444-63929-5.00002-4
- ↑ 4.0 4.1 Stewart, L. (1998). A Model for the Mechanism of Human Topoisomerase I. Science, 279(5356), 1534–1541. https://doi.org/10.1126/science.279.5356.1534
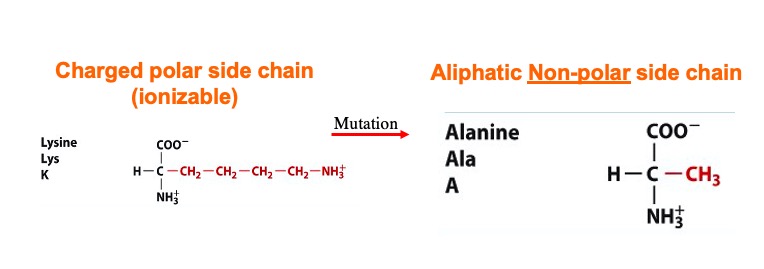
- ↑ 5.0 5.1 Interthal H, Quigley PM, Hol WG, Champoux JJ. The role of lysine 532 in the catalytic mechanism of human topoisomerase I. J Biol Chem. 2004 Jan 23;279(4):2984-92. Epub 2003 Oct 31. PMID:14594810 doi:10.1074/jbc.M309959200