Sandbox Reserved 1086
From Proteopedia
| This Sandbox is Reserved from 15/04/2015, through 15/06/2015 for use in the course "Protein structure, function and folding" taught by Taru Meri at the University of Helsinki. This reservation includes Sandbox Reserved 1081 through Sandbox Reserved 1090. |
To get started:
More help: Help:Editing |
Contents |
C-cadherin
| |||||||||||
Exploring the Structure
The extracellular moiety of the protein consists of five ectodomains () alternated by four . Each EC shares the same greek key fold, characteristic of immunoglobulins (Fig.1). Each interface between the EC is a calcium binding site, coordinating three ions through the highly conserved motifs Asp-X-Asp, Leu-Asp-Arg-Glu, and Asp-X-Asn-Asp-Asn. The binding of calcium is thought to confer rigidity as well as the classical curved shape to the structure. This is why it´s suggested to be a triggering step to the adhesion process,if not even compulsory [4].
Calcium Coordination
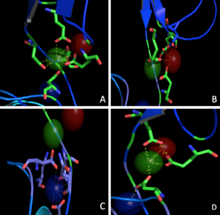
Three calcium ions are differently coordinated in each interface between two neighbouring EC. Just one of these seems to be coordinated with the classical epta-coordination characteristic of the elements belonging to the first and second group (Fig.2A) while the others are bound without any particular geometry (Fig.2B,2C). Nevertheless, the presence of common coordinating residues (Glu) links these ions one to another forming a net of strong interactions (Fig.2D).
Molecular Geometry
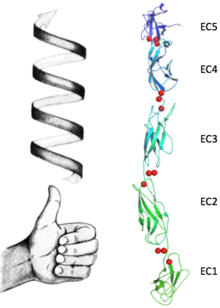
Upon the calcium binding each EC contracts to its neighbour, resulting in a defined position which leads to the bended structure of the protein. These conformations changes in a sequel of ions form the unique final conformation of this protein. In Fig.3, the bending between EC1 and EC2 is shown in just one dimension, making clear the angle between the two main axes (approximately 22.5°). Fig.4, represents the bending between EC2 and EC3 while concealing this property between first and second EC. Because of this concealing (due to the change in the observed direction), the change of position of EC3 with respect EC2 can no longer be addressed in the same direction of the bending between EC1 and EC2. This occurrence of bendings in (at least) two different directions results in a torsional behaviour which goes through all the EC. These particular fashion contributes to the complexity of the protein´s ternary structure, shaping it into a bended left handed helix (Fig.5).
Adhesion Models
This conformation (called S) might play a key role in tackling the pulling forces within the desmosomal plaque. In fact, two cadherins that take contact from two different surfaces are going to perpetuate the left handed shape to the quaternary structure. As a consequence, the dimer unit connecting the two cells will resist to the stretching force with the same features of a spring (Fig.5). The S shape conformation shown in this model is not the only one observed. Experiments of electron microscopy [5] have identified different molecular species corresponding to the cadherin dimer. One of these is called W (1q55), in which cadherins partecipate to the binding by burying the 2 into the hydrophobic pocket (HAV sequence) present on the EC1 of the facing molecule [4]. Depending on the shape adopted by the dimer, these Trp2 have been seen to promote the cis interaction as well. In fact in the S shape, although one 2 would remain in its partner´s hydrophobic pocket, the other one would be pulled out of the pocket and become free for interaction with additional neighbouring cadherins. This trimeric conformation is known as Y shape (1q5b).
Glycosylation
C cadherin is a N/O-glycosilated protein. It counts 15 sugars (, ) attached all over the structure (mainly to EC3 and EC4) to Thr-Asn residues. Their presence has been proved to affect the dynamics of the adhesion process and specifically the formation rate of the cis interactions [6]. By contrast the kinetics of EC1-EC1 trans interaction for glycosilated and hypo-glicosilated cadherins remains the same. These data offer several interpretations for the biological role of glycans. One of these, proposes that the steric interference of N-glycans might alter the free energy difference between monomers and dimers shifting their equilibrium [7]. This would create a kinetic barrier that would slow the (cis) dimerization rate, favouring the EC1-EC1 (trans) interaction.
Structural highlights
| 1q5a is a 2 chain structure with sequence from Mus musculus. The March 2008 RCSB PDB Molecule of the Month feature on Cadherin by David S. Goodsell is 10.2210/rcsb_pdb/mom_2008_3. Full crystallographic information is available from OCA.
This is a view if the molecule by groups, and the of the protein.
For a guided tour on the structure components use FirstGlance. | |
| Ligands: | , , |
| Related: | 1l3w, 1q55, 1q5b, 1q5c |
| Resources: | FirstGlance, OCA, RCSB, PDBsum |
References
- ↑ Basu R, Taylor MR, Williams ME. The classic cadherins in synaptic specificity. Cell Adh Migr. 2015 Apr 2:1-9. PMID:25837840 doi:http://dx.doi.org/10.1080/19336918.2014.1000072
- ↑ Fulga V, Rudico L, Balica AR, Cimpean AM, Saptefrati L, Margan MM, Raica M. Differential expression of e-cadherin in primary breast cancer and corresponding lymph node metastases. Anticancer Res. 2015 Feb;35(2):759-65. PMID:25667455
- ↑ Delva E, Tucker DK, Kowalczyk AP. The desmosome. Cold Spring Harb Perspect Biol. 2009 Aug;1(2):a002543. doi:, 10.1101/cshperspect.a002543. PMID:20066089 doi:http://dx.doi.org/10.1101/cshperspect.a002543
- ↑ 4.0 4.1 Boggon TJ, Murray J, Chappuis-Flament S, Wong E, Gumbiner BM, Shapiro L. C-cadherin ectodomain structure and implications for cell adhesion mechanisms. Science. 2002 May 17;296(5571):1308-13. Epub 2002 Apr 18. PMID:11964443 doi:http://dx.doi.org/10.1126/science.1071559
- ↑ He W, Cowin P, Stokes DL. Untangling desmosomal knots with electron tomography. Science. 2003 Oct 3;302(5642):109-13. PMID:14526082 doi:10.1126/science.1086957
- ↑ Langer MD, Guo H, Shashikanth N, Pierce JM, Leckband DE. N-glycosylation alters cadherin-mediated intercellular binding kinetics. J Cell Sci. 2012 May 15;125(Pt 10):2478-85. doi: 10.1242/jcs.101147. Epub 2012, Feb 17. PMID:22344255 doi:http://dx.doi.org/10.1242/jcs.101147
- ↑ Guo HB, Johnson H, Randolph M, Pierce M. Regulation of homotypic cell-cell adhesion by branched N-glycosylation of N-cadherin extracellular EC2 and EC3 domains. J Biol Chem. 2009 Dec 11;284(50):34986-97. doi: 10.1074/jbc.M109.060806. Epub, 2009 Oct 21. PMID:19846557 doi:http://dx.doi.org/10.1074/jbc.M109.060806