Sandbox Reserved 1101
From Proteopedia
| This Sandbox is Reserved from 25/11/2019, through 30/9/2020 for use in the course "Structural Biology" taught by Bruno Kieffer at the University of Strasbourg, ESBS. This reservation includes Sandbox Reserved 1091 through Sandbox Reserved 1115. |
To get started:
More help: Help:Editing |
N-terminal domain of Major-ampullate Spidroin protein
5IZ2 is the N-terminal domain (NTD) of a spider protein called Major ampullate Spidroin 1A (MaSp1A), coming from the Nephila Clavipes species. This protein is a component of dragline silk produced in the major ampullate gland of spiders[1]. The NTD domain of MaSp1A plays a major role in their combination during silk production [1]. Indeed, thanks to the NTD dimerisation, two MaSps can be connected, leading to the formation of fibers with exceptional physical and biochemical qualities [2]. It is of biotechnological interest to deeply understand the NTD dimerisation mechanism for the production of artificial spider silk, which can lead to innovative biomaterials. The study of the N. Clavipes NTD permits to compare its structure with other species thus to provide new insights into the mechanism of NTD dimerization. Moreover, silks produced from different spider breeds vary in physical properties such as toughness and elasticity. In this way, studying diverse species would allow to optimize artificial silk for different applications.
Contents |
Generalities on fiber assembly of dragline silks
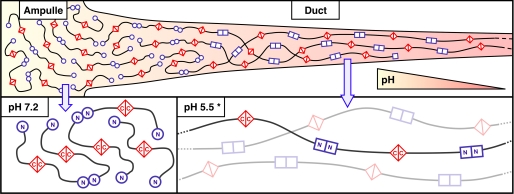
The process of the dragline fiber formation is the connection of soluble MaSp proteins into insoluble fibers. Indeed, MaSps are firstly secreted and stored in soluble form in the tail of the major ampullate gland which is located in the spider’s abdomen. On demand, they pass through the narrow duct where they experience mechanical and chemical forces that convert them into fibers.
Actually, they deal with a pH dropping, an alteration of ion concentrations and oxidation conditions, which occur gradually along the duct. These changes promote the connection of MaSps extremities (i.e. homo-dimerisation of C- and N-terminal domains) to form fibers. Finally, through flow rate and mechanical forces experienced in the duct, the fibers will agglomerate to create the dragline silk.
Overall structure of Major-ampullate Spidroin protein
The dragline fiber is mainly composed of proteins termed Major ampullate Spidroin 1 and Major ampullate Spidroin 2 (MaSp1 and MaSp2). MaSp1 is found in both the core and periphery of the fiber, while MaSp2 is only assembled in the core [3]. In Nephila clavipes, there are two distinct MaSp1 genes ; MaSp1A and MaSp1B [4]. The MaSps are between 250 to 350 kDa [3]. They are divided into three parts : C-terminal domain (CTD), repeat domain (RD), and N-terminal domain (NTD).
- Repeat domain (RD):
The MaSp sequence corresponds to more than 90% of RD [5]. The RD is a long, flexible, highly repetitive central domain. It varies greatly between the types of silks, which makes it responsible for their different properties. MaSp1 contains poly-alanine (A)n motifs at the end of a repeat, as well as GA and GGX motifs where X is often A, Y, L, or Q [3]. The poly-alanine motifs, usually present at the end of a repeat, form β-sheets in the duct due to mechanical forces. The β-sheets will then line up in parallel, leading to the aggregation of the fibers. The GGX motifs form an amorphous matrix that connects the crystalline regions [6].
- C-terminal domain (CTD):
The CTD is a non-repetitive sequence of about 150 amino acids [3]. The sequence identity, secondary structure and overall physical properties of CTD is highly conserved across spider species [3]. Its structure forms a bundle of five parallel α-helices. A single cysteine residue in the middle of its sequence is highly conserved and is responsible for the CTD homo-dimerisation. In other words, it allows the covalent connection between two CTDs through disulfide bond linkage. The CTD also plays a role in the change of MaSps solubility according to its localisation in the gland [3]. Indeed, it presents a high number of charged and polar amino acids present in its sequence. In this way, when the pH is neutral in the ampullate, the hydrophobic residues are buried within the core and the hydrophilic residues are exposed. This permits to keep the MaSps soluble, preventing early fiber aggregation. On the contrary, when the CTDs are in the duct with lower pH, the acidic residues switch from a negative to a neutral charge. This leads to an increase of hydrophobic interactions that help with the formation of β-sheets and thus MaSps precipitation.
- N-terminal domain (NTD):
This domain is the most highly conserved domain. NTD dimerises in the duct upon conditions change, which connects the MaSps to form fibers.
Monomer structure of the spidroin NTD domain
|
One monomer of NTD (N-Terminal Domain) is composed of 5 parallel α-helix ()[3].
In each subunit, the orientation of helices 2, 3 and 5 is different from the orientation of helices 1 and 4. Indeed, helices 1 and 4 form the rigid body of the NTD domain, while helices 2, 3 and 5 are involved in intermolecular contacts, so they play an important role in the dimerization process[1].
Moreover, at the opposite extremities of each subunits of the monomer there are clusters of acidic residus (Asp36, Asp39, Asp40, Glu79, Asp91) in one part, and clusters of basic residus (Lys54, Arg57, Lys60, Lys64, Lys65) in the other part. In addition to this, the subunits A and B are organized antiparallel, which allows an access to charges poles. The charged residues (the acidic and basic ones) are responsible for creating a dipole moment, which therefore implies a non-uniform charge arrangement within the subunits. This is important for the dimerization process, that is why they are highly conserved residues[1].
Compared with spidroin of other species of spider, the 2 subunits (A and B) of the dimerized NTD of the spidroin produced by N. Clavipes are slightly different, due to a different helices arrangement. So they do not completely overlap. This allows the creation of new intermolecular contact networks. There is also a composed of 3 amino acids (Ser, Tyr, Gly), but it role is not well established yet[1].
Dimerization of the spidroin by the NTD domain
Conformational change of the five-helix bundle
The dimerization of the spidroin by the NTD domain begins by a rearrangement of the five-helix bundle during the monomer to dimer transition. An acidification along the spinning duct results in a conformational change of the NTD. So, for the NTD dimerization, a lowering of pH from 7 to 6 is important. Then, a subunit selects a partner with a complementary binding interface.[1] When the NTD forms a dimer, its positive and negative poles are opposed, creating an environment conducive to salt bridges formation.[3] Moreover, dimerization is really triggered and stabilized by protonation of some residues. Studies have also shown that a lowering more important of the pH stabilizes even more the dimer. The plasticity of the dimer interface could also be a factor of the conformational selection during transition from monomer to dimer or during the transition from loosely to stably dimer. [1]
Interactions
|
Principal interactions
Different types of interactions occur between specific residues during the NTD dimerization. Asp40, Lys65, Asp39 and Glu84 residues have been identified as being particularly important. In one side, engage in the intramolecular handshake interaction. The asymmetric nature and the difference of topology of the subunits allow the formation of salt bridges. engage in a short-range intermolecular salt bridge of 2,6 Å. In the other side, engage in a short-range intermolecular salt bridge of 3,1 Å. Asp39 is not involved in this part of the dimer. The structure of N. clavipes dimer interface differs from those of other species due to the asymmetric nature of the interface and the involvement of Asp39. It has been reported that Asp39 is essential for the NTD dimerization in other species of spiders and seems to be also important in N.clavipes. These interactions make subunits alignment better. Acidic residues are conserved around residues Asp39 and Asp40 and this allows the variability in the interactions that take place to Lys65. This variability provides a mechanism for plasticity in the dimer interface allowing the transition from loosely to stably associated dimer [1]
Another intramolecular handshake interaction occurs also between . This interaction doesn’t exist in subunit B because of the orientation of subunit A with respect to subunit B, Asp17 and Asp53 are too far away in order to engage this interaction. [1]
Secondary interactions
These asymmetric contacts play a well-defined role in dimer formation in many species of spiders but in N. clavipes several other novel interactions occur. For example, in comparison with the Euprosthenops australis NTD, N. clavipes NTD engage more than 38,5% of novel interactions. These ones result from the distinct topology of the three helices (H2, H3 and H5) compared to other species. Indeed, the specific angles at which the H2, H3 and H5 helices cross their counterparts in the asymmetric interface allow the correct positioning of residues and the establishment of these interactions. [1]
- Van der Waals
Residues T47B, M55B and K54B are more buried at the dimer interface creating specific contacts. . Also, . In subunits H2A and H2B, T47A and A51B engage in a Van Der Waals interaction of 4,1 Å, and that contribute to the plasticity of the dimer interface.[1]
- Hydrogen bonds and electrostatic interactions
K54B engage in a unique hydrogen bond to and electrostatic interaction with . [1]
On the other side on the dimer interface, there are also other specific contacts but distinct due to the different topology. But residues T47A, K54A and M55A are less buried than their counterparts in subunit A in particularly K54A which doesn’t engage any interaction. [1]
- Hydrophobic pockets
Then, in subunits H5A and H5B, and also buried at the dimer interface, insert into hydrophobic pockets formed by S122B, L123B and M71B, S75B, E119B and I120B respectively. [1]
pH-dependent mechanism
In order to observe the pH-dependent NTD dimerization mechanism, a tryptophan fluorescence assay was used. The N. clavipes NTD contains a single tryptophan (Trp10) near the N-terminus. During the transition from the NTD monomer to the NTD dimer, a conformational change occurs for Trp10 that increases its solvent exposure. As a consequence, a quenching of its fluorescence emission is observed. The transition from the NTD monomer to the NTD dimer occurs at pH 6,1'. At pH above 6,1, NTD is in the form of monomer and the formation of dimer occurs after pH 6,1. Mutations in residues Asp40, Lys65 involved in salt bridges result in decrease in dimer stability. This assay shows that short-range asymmetric salt bridges between Asp39, Asp40 and Lys65 are essential to the NTD dimerization. Next, a mutation of residue Glu84 completely destabilize the dimer formation, that shows the importance of the handshake interaction and also the protonation of Glu84, which must be preceded by protonation of Glu79 and Glu119. Similarly, the protonation of Asp17 and Asp53 plays also a key role in the mechanism of NTD dimerization [1]. These protonations are allowed by the lowering of the pH suffered by the NTD during its progression in the spinning duct [3].
Applications in Biotechnology
The dragline silk represents the “toughest biopolymer on Earth” [7]. It also shows other beneficial properties including high tensile strength, elasticity and biodegradability. That being, dragline fibers can have many uses in medical and industrial fields.
Synthetic silk proteins are commonly produced by recombinant gene expression and gene mimicry [8]. They can be spontaneously optimised by altering their form, size and composition. Indeed, DNA sections in silk protein sequence can be rearranged, added to or subtracted from to change the characteristics of the formed protein. For instance, silk proteins can be processed into many different forms such as fibers, sponges, films, capsules and gels [3]. Their biodegradability can also be altered as required to increase or reduce their degradation time [3]. In this way, the uses for spider silk can give rise to a wide range of novel materials.
As far as the medical field is concerned, spider silk is naturally biocompatibility. This allows its use for applications like drug release materials, cell graft scaffolds, neuron regeneration and cartilage repair [3]. Moreover, the spider silk can be recombinantly engineered to produce antimicrobial propertiers, certainly useful in this sector [9].
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 Atkison JH, Parnham S, Marcotte WR, Jr., and Olsen SK, 2016. Crystal Structure of the Nephila clavipes Major Ampullate Spidroin 1A N-terminal Domain Reveals Plasticity at the Dimer Interface, The Journal of Biological Chemistry, vol.291 no.36, p.19006-19017.
- ↑ José Roberto Aparecido dos Santos-Pinto, Helen Andrade Arcuri, Helga Priewalder, Heliana Clara Salles, Mario Sergio Palma and Gert Lubec, 2015. Structural Model for the Spider Silk Protein Spidroin‑1, Journal of Proteome research, 14, p.3859-3870.
- ↑ 3.00 3.01 3.02 3.03 3.04 3.05 3.06 3.07 3.08 3.09 3.10 3.11 Cadle KA, 2016. “The Role the N-terminal Domain Plays in Spidroin Assembly”, All Dissertations. 2296 https://tigerprints.clemson.edu/all_dissertations/2296/?utm_source=tigerprints.clemson.edu%252Fall_dissertations%252F2296&utm_medium=PDF&utm_campaign=PDFCoverPages.
- ↑ Gaines WA 4th, Marcotte WR Jr. Identification and characterization of multiple Spidroin 1 genes encoding major ampullate silk proteins in Nephila clavipes. Insect Mol Biol. 2008 Sep;17(5):465-74. doi: 10.1111/j.1365-2583.2008.00828.x. PMID:18828837 doi:http://dx.doi.org/10.1111/j.1365-2583.2008.00828.x
- ↑ Romer L, Scheibel T. The elaborate structure of spider silk: structure and function of a natural high performance fiber. Prion. 2008 Oct-Dec;2(4):154-61. doi: 10.4161/pri.2.4.7490. Epub 2008 Oct 20. PMID:19221522 doi:http://dx.doi.org/10.4161/pri.2.4.7490
- ↑ Huemmerich D, Scheibel T, Vollrath F, Cohen S, Gat U, Ittah S. Novel assembly properties of recombinant spider dragline silk proteins. Curr Biol. 2004 Nov 23;14(22):2070-4. doi: 10.1016/j.cub.2004.11.005. PMID:15556872 doi:http://dx.doi.org/10.1016/j.cub.2004.11.005
- ↑ Tokareva O, Michalczechen-Lacerda VA, Rech EL, Kaplan DL. Recombinant DNA production of spider silk proteins. Microb Biotechnol. 2013 Nov;6(6):651-63. doi: 10.1111/1751-7915.12081. PMID:24119078 doi:http://dx.doi.org/10.1111/1751-7915.12081
- ↑ Humenik M, Scheibel T, Smith A. Spider silk: understanding the structure-function relationship of a natural fiber. Prog Mol Biol Transl Sci. 2011;103:131-85. doi:, 10.1016/B978-0-12-415906-8.00007-8. PMID:21999996 doi:http://dx.doi.org/10.1016/B978-0-12-415906-8.00007-8
- ↑ Gomes SC, Leonor IB, Mano JF, Reis RL, Kaplan DL. Antimicrobial functionalized genetically engineered spider silk. Biomaterials. 2011 Jun;32(18):4255-66. doi: 10.1016/j.biomaterials.2011.02.040., Epub 2011 Mar 31. PMID:21458065 doi:http://dx.doi.org/10.1016/j.biomaterials.2011.02.040