This is a default text for your page Blake Moskal/Sandbox1. Click above on edit this page to modify. Be careful with the < and > signs.
You may include any references to papers as in: the use of JSmol in Proteopedia [1] or to the article describing Jmol [2] to the rescue.
Introduction
Human GPR40 receptor, hGPR40, is a free fatty-acid receptor that binds to long chain free fatty acids, inducing insulin secretion. However, what makes this receptor significant is that the secretion of insulin is glucose dependent. Thus, there needs to be an agonist bound, in addition to presence of glucose in the blood in order for insulin secretion to occur. This glucose-dependence makes GPR40 a target for type-2 diabetes because it allows for increased glycemic control and therefore, low risk of hypoglycemia.
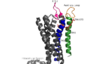
Structure
Transmembrane Proteins
Extracellular Loops
Disulfide Bond
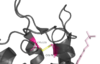
TAK-875 Binding
Tak-875 is known to be a partial agonist of GPR40. The bonding of this ligand to the bonding site is fairly unique, as it is proposed that the ligand must enter through the membrane bilayer. This is performed via a method similar to ligand binding to sphingosine 1-phosphate receptor 1 3v2w, retinal loading of opsin 4j4q and the entry of anandamide in cannabinoid receptors, in which extracellular loops block the binding from the extracellular matrix (Hanson Et al., 2012). In contrast, delta opioid receptor binding 4ej4 allow for binding directly from the extracellular matrix. The binding mechanism through the bilayer may be selectively favoring the free fatty acid because of the non-polar regions of the ligand.
Disease
Relevance
Structural highlights
This is a sample scene created with SAT to by Group, and another to make of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes.