Sandbox Reserved 1283
From Proteopedia
Contents |
genetics is ok
'Molecules it Interacts With and where '
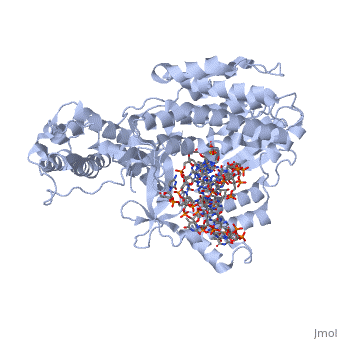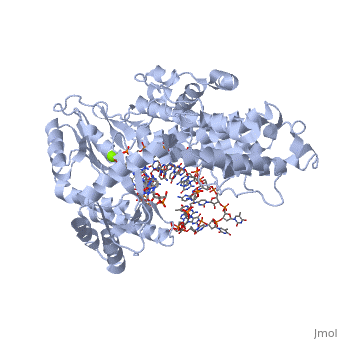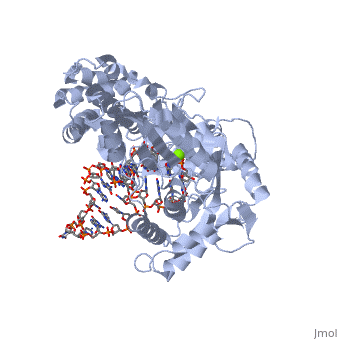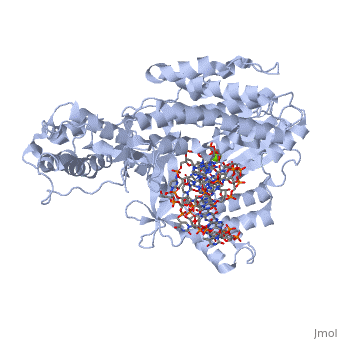
The protein binds to GDP as well as the following ligands in order to promote the attachment of the protein complex to the ribosome A site.
PHOSHOAMINOPHOSPHONIC ACID-GUANYLATE ESTER
PHENYLALANINE
MAGNESIUM ION
'Origin'
It has domains that are created in yeast (phenyl-transfer RNA) , in the heat resistant Thermus aquaticus (EF-Tu elongation factor, and can be synthetically manufactured.
'Structure'
It has 3 domains. G proteins, Elongation Factors, and the EF-Tu/eEF-1alpha/eIF2-gamma C-terminal domain. It is composed of 6 chains, which combine in alignment.
Specific are highlighted here. The ligands listed above, GDP, Phe, and Mg+2 ion each attach at these locations which are still being explored.
which play a crucial role in binding to the ribosome during translation. They form positive pockets with which negative amino acids can bind to.
'Molecules it Interacts With and where '
The protein binds to GDP as well as the following ligands in order to promote the attachment of the protein complex to the ribosome A site.
PHOSHOAMINOPHOSPHONIC ACID-GUANYLATE ESTER
PHENYLALANINE
MAGNESIUM ION
'Origin'
It has domains that are created in yeast (phenyl-transfer RNA) , in the heat resistant Thermus aquaticus (EF-Tu elongation factor, and can be synthetically manufactured.
'Structure'
It has 3 domains. G proteins, Elongation Factors, and the EF-Tu/eEF-1alpha/eIF2-gamma C-terminal domain. It is composed of 6 chains, which combine in alignment.
Specific are highlighted here.
which play a crucial role in binding to the ribosome during translation.
'Function"
The protein complex participates in placing the amino acids in their correct order when messenger RNA is translated into a protein sequence on the ribosome by promoting GTP-dependent binding of tRNA to the A site of the ribosome. In other words, it is involved with elongation during polypeptide synthesis.
| |||||||||||
Telomerase
Telomerase adds a repetitive sequence called a telomere to the ends of eukaryotic chromosomes in most eukaryotes. Telomeres are short, repetitive DNA sequences at chromosome ends that protect the genetic information from deterioration. They are also rich in .
|
Structure
Telomerase is composed of two molecules each of human telomerase reverse transcriptase (TERT), telomerase RNA (TR or TERC), and dyskerin (DKC1). TERT has a mitten structure that allows it to wrap around the chromosome and add nucleotides. An is used to transcribe its own RNA sequence into DNA which it adds on to the end of the chromosome.
What It Interacts With
RNA sequence template in telomerase hybridizes with single stranded overhang on DNA strand leaving RNA overhang. DNA polymerase function of telomerase adds complemetary nucleotides to RNA overhand which extends the telomere.[1]
Where It Interacts
DNA polymerase can only extend DNA in the 3’ direction with an RNA primer. At the 3’ end of a template strand, space for the RNA primer eventually runs out, creating an overhanging edge of DNA that cannot be completed.
Telomerase contains a strand of RNA that partially attaches to the overhanging 3’ end of the template strand. DNA polymerase uses this RNA template to extend the template strand’s . This allows space for DNA primase to add a primer for the nontemplate strand so DNA polymerase can copy the nucleotides that were previously missed.
Where It Originates
This process occurs in the nucleus during DNA replication.
Telomerase is found particularly in fetal cells, adult stem cells, and tumor cells.
Over time, the amount of telomerase on chromosomes decreases, leading to signs of aging.
References
- ↑ https://highered.mheducation.com/sites/9834092339/student_view0/chapter14/telomerase_function.html
You may include any references to papers as in: the use of JSmol in Proteopedia [1] or to the article describing Jmol [2] to the rescue.