This is our page on hemoglobin.
Function
Hemoglobin in vertebrates carries oxygen from the respiratory organs (lungs and gills) to the rest of the body tissues. About 270 million hemoglobin molecules reside in each red blood cells, which are circulated to use it to carry oxygen around the bodies of vertebrates. Hemoglobin is also what causes their blood to appear red.
Structural Highlights
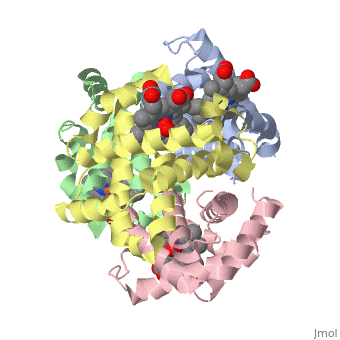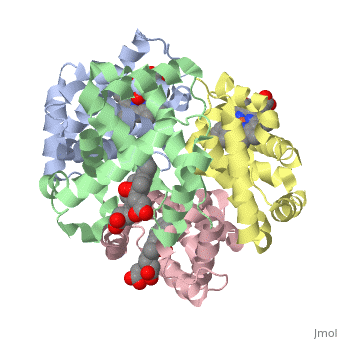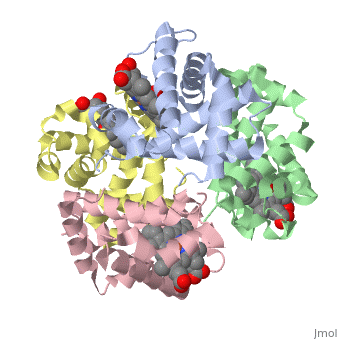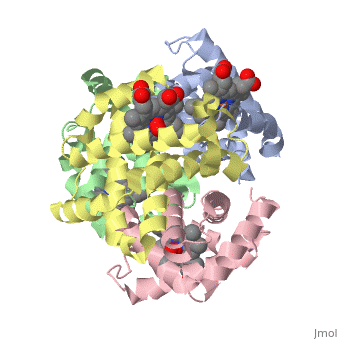
Hemoglobin is made of four protein chains. There are two and two chains in each molecule of hemoglobin. Each chain looks like myoglobin, which is used to store oxygen in muscles. Each individual chain has a that has an iron atom that allows oxygen to bind. Of the chains, two are beta chains and two are alpha chains.
In the chain, the heme group has iron that allows the oxygen to bind.
Diseases
Sickle Cell Anemia
For the most part, amino acid sequences can be slightly different, but sometimes a change in the sequence can have a large impact. For example, in sickle cell anemia, glutamate 6 in the beta chain is mutated to valine, resulting in a structural change that allows hemoglobin to stick to each other and create stiff fibers, which creates the sickle shape in blood cells. These fibers are helpful because the malaria virus cannot live in blood cells with them, but can also lead to rupture of the blood cell, causing a loss of hemoglobin. The sickle cell mutation typically affects one of the , causing an abnormal beta chain called Hbs.
Thalassemia
This is another genetic disease involving hemoglobin. Here, there is some sort of error in the production of beta proteins. In normal hemoglobin, there is two alpha proteins and two beta, but when thalassemia occurs, there may be low levels or no , decreasing the amount of functional hemoglobin and therefore supplies of red blood cells, often causing anemia.
References
https://ghr.nlm.nih.gov/condition/beta-thalassemia#genes
http://pdb101.rcsb.org/motm/41
https://ghr.nlm.nih.gov/condition/sickle-cell-disease#genes