Sandbox Reserved 1408
From Proteopedia
| This Sandbox is Reserved from January through July 31, 2018 for use in the course HLSC322: Principles of Genetics and Genomics taught by Genevieve Houston-Ludlam at the University of Maryland, College Park, USA. This reservation includes Sandbox Reserved 1311 through Sandbox Reserved 1430. |
To get started:
More help: Help:Editing |
Contents |
Structure
|
Protein p53 is made of 393 amino acids, consisting of seven domains. These domains include proline rich domain, activation domain 2, transcription activation domain (14 amino acids), DNA-binding core domain (198 amino acids), C-terminal domain, tetramerzatin domain (30 amino acids), and a nuclear localization signaling domain. These are the of protein p53. These are the of protein p53.
Function
Protein p53 is a transcription factor that regulates DNA repair, cell arrest, and apoptosis by . It is activated in response to stressors including radiation, oxidation stress, porto-oncogenes, and DNA-damaging agents.
Significance
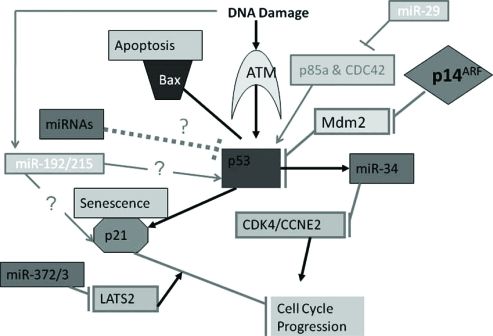
One example of p53 function is causing cell arrest. When DNA damage is detected, kinase ATM will increase p53 levels. High p53 levels may induce p21 which will ultimately stop cell cycle progression. Additionally, p53 is a tumor suppressor gene. If the p53 gene is not functional in an individual, then that individual will be predisposed to developing cancer because there will be a lack of cell and DNA repair regulation.
References
akwi, A., & Li, Y. (2009). The p53 Pathway Encounters the MicroRNA World. Current Genomics, 10(3), 194–197. http://doi.org/10.2174/138920209788185270
National Center for Biotechnology Information (US). Genes and Disease [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 1998-. The p53 tumor suppressor protein. Available from: https://www.ncbi.nlm.nih.gov/books/NBK22268/