Sandbox Reserved 1471
From Proteopedia
| This Sandbox is Reserved from November 5 2018 through January 1, 2019 for use in the course "CHEM 4923: Senior Project taught by Christina R. Bourne at the University of Oklahoma, Norman, USA. This reservation includes Sandbox Reserved 1471 through Sandbox Reserved 1478. |
To get started:
More help: Help:Editing |
This page is reserved for Katie
Contents |
Cyclooxygenase 2
|
A Cyclooxygenase is an enzyme that catalyzes the transformation of arachidonic acid into prostaglandins, prostacyclins, and thromboxanes.[1] Another name for cyclooxygenase is prostaglandin H2 synthase. [2] There are two names because there are two catalytic activities: cyclooxygenase and peroxidase. The abbreviation for cyclooxygenase and prostaglandin H2 synthase is COX and PGHS respectively and can be used interchangeably. There are two types of cyclooxygenases: COX-1 and COX-2. COX-1 is responsible for platelet aggregation and gastric acidity.[1] COX-2 is involved in pathways that lead to inflammation (swelling), pain, and fever.[1]
Function
Reaction
Cyclooxygenases catalyze arachidonic acid or other fatty acids into prostaglandin H2 (PGH2) and other molecules that can be used for signal transduction. Of the two catalytic activities, the cyclooxygenase reaction happens before the peroxidase reaction. However, the peroxidase activity activates the cyclooxygenase activity.[3] “Two-electron reduction of a peroxide substrate results in the oxidation of the ferric heme to an oxo-ferryl porphyrin radical cation.”[4] The most important catalytic residue is . It transfers an electron to the heme to create the radical on the Tyrosine [4] The Tyrosine then takes the pro-S hydrogen from carbon 13 of arachidonic acid to produce a radical on the arachidonic intermediate. [5] Two oxygen molecules are inserted to cyclize the intermediate.[5] Tyrosine 385 is reduced from the “peroxyl radical to the hyperoxide to form PGG2.”[4] PGG2 is then reduced by the peroxidase activity to form PGH2.[5] The Tyrosine 385 radical is regenerated, so the cyclooxygenase activity does not need to be activated for every reaction.[4]
Medical Applications
There are many medical applications for the cyclooxygenase isozymes. COX-1 is responsible for platelet aggregation and gastric acidity.[1] COX-2 is involved in pathways that lead to inflammation (swelling), pain, and fever.[1] Inhibiting either or both of these enzymes can be beneficial. Inhibiting COX-1 for a brief period can reduce platelet aggregation, but if COX-1 is inhibited too long, gastric damage such as ulcers and bleeding can occur.[1] Inhibitors that bind specifically to COX-1 or inhibitors that bind nonspecifically to the cyclooxygenases need to be small dosages and not for elongated periods of time. Inhibiting COX-2 can reduce swelling, pain, and fever.[1] However, COX-2 is involved in both the initiation of inflammation and the resolution of inflammation, so it cannot be inhibited for long periods of time either, in the chance that the inflammation resolution will not occur.[2] Both isozymes are expressed in the female reproductive system.[2] A study on rats revealed that overexpression of the COX-2 enzyme increased the chance of tumor development, during pregnancy and lactation, in mammary glands.[6] COX-2 is expressed when it is induced from a signal molecule while COX-1 is constitutively expressed.[2] Both isozymes can be expressed in the same cell, but COX-2 will be predominately active since it is inducible and COX-1 is always expressed.[2]
Structure
Domains
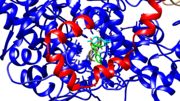
COX-1 is made up of 576 amino acids and COX-2 is made up of 581 amino acids.[4] The sequences match with 60% identical amino acids.[4] The structures of these isozymes are nearly superimposable with three domains each. The made up of residues 73-116, goes through half of the lipid bilayer.[3] These residues are hydrophobic because they are in a hydrophobic environment with the fatty acid tails of the lipids surrounding this domain. Residues 117-587 make up the .[3] The catalytic domain has two catalytic active sites for each of the catalytic activities of cyclooxygenase and peroxidase. The third domain is similar to an .[3] The function of the EGF domain is not fully understood, but it is speculated to help with structure stability and protein-protein interactions.[3] This domain is linked to the catalytic domain by a disulfide bridge between Cys 37- Cys 159.[3]
Reaction
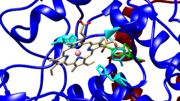
Arachidonic acid can bind in the nonproductive conformation and the productive conformation.[7] In the nonproductive conformation, the carboxyl of arachidonic acid is interacting with Tyrosine 385 and Serine 530.[7] The productive conformation has the carboxyl of arachidonic acid interacting with Arginine 120 and Tyrosine 355 which leaves Tyrosine 385 available to take the pro-S hydrogen from carbon 13.[7] In other words, the productive conformation yields a reaction and product while the nonproductive conformation does not. In figure 2 and 3, Tyrosine 385 is colored in cyan. These figures show how the residue is in the middle of the cyclooxygenase channel and can easily be inhibited by Rofecoxib blocking the cyclooxygenase active site. In these images, the membrane binding domain is colored in red and the catalytic domain is colored in blue. Figure 3 shows the peroxidase active site with the protoporphyrin IX containing cobalt. Other structures showed a heme group interacting with COX. There does not seem to be a preference over the protoporphyrin verses the heme group. Sticking with the PDB file of 5KIR, the proximal Histidine 388 and the distal Histidine 207 coordinate the protoporphyrin group colored in cyan.[1]
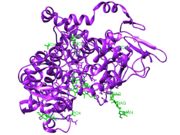
Heteroatoms of the PDB file 5KIR[1]
The heteroatoms can be seen in green in Figure 1.
| Heteroatom Name | ID |
|---|---|
| Phosphate Ion | PO4 |
| Rofecoxib | RCX |
| Glycerol | GOL |
| Alpha-D-Mannose | MAN |
| Protoprophyrin IX Containing Cobalt | COH |
| Ammonium Ion | NH4 |
| N-Acetyl-D-Glucosamine | NAG |
Energetics
Arachidonic acid has a higher kcat value of 27.0 ± 0.4 s-1 and a lower Km value of 5.14 ± 0.29 µM than EPA and DHA for the affinity to COX-2.[7] This means that arachidonic acid is the preferred substrate for COX-2 when compared with EPA and DHA fatty acids. The rate of the reaction can depend on the concentration of the substrate. However, the rate can be slowed or stopped with an inhibitor. and Celecoxib are two inhibitors that have selectivity towards COX-2.[1] They differ from the nonsteroidal anti-inflammatory drugs (NSAIDs) because of their selectivity for COX-2.[1] NSAIDs inhibit both COX-1 and COX-2.[1] Rofecoxib, commonly known as Vioxx, has a 1000-fold selectivity for COX-2 over COX-1.[8] It also has a 60-fold selectivity for COX-2 when compared to the selectivity of Celecoxib (Celebrex) for COX-2.[1] The difference in the kinetics of Rofecoxib and Celecoxib is speculated to be from the binding kinetics and not the interaction between the inhibitor and the enzyme.[1] The rate constant for the inhibition of COX-2 by Rofecoxib was experimentally determined to be 0.0036 ± 0.0024 µM-1s-1 for a two-step mechanism.[8] When bound to COX-2, Rofecoxib makes 42 contacts with the nearby residues where it binds.[1] Celecoxib differs from Rofecoxib in structure by having a “pyrazole heterocycle and a sulfonamide moiety” whereas Rofecoxib has a “furanone heterocycle and a methyl sulfone moiety.”[1]
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 Orlando BJ, Malkowski MG. Crystal structure of rofecoxib bound to human cyclooxygenase-2. Acta Crystallogr F Struct Biol Commun. 2016 Oct 1;72(Pt 10):772-776. Epub 2016, Sep 22. PMID:27710942 doi:http://dx.doi.org/10.1107/S2053230X16014230
- ↑ 2.0 2.1 2.2 2.3 2.4 Smith, W. L and R. Langenbach (2001). "Why there are two cyclooxygenase isozymes." The Journal of Clinical Investigation 107(12):1491-1495.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 Picot D, Loll PJ, Garavito RM. The X-ray crystal structure of the membrane protein prostaglandin H2 synthase-1. Nature. 1994 Jan 20;367(6460):243-9. PMID:8121489 doi:http://dx.doi.org/10.1038/367243a0
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 Rouzer CA, Marnett LJ. Cyclooxygenases: structural and functional insights. J Lipid Res. 2009 Apr;50 Suppl:S29-34. doi: 10.1194/jlr.R800042-JLR200. Epub 2008, Oct 23. PMID:18952571 doi:http://dx.doi.org/10.1194/jlr.R800042-JLR200
- ↑ 5.0 5.1 5.2 "Chapter 21: Lipid Biosynthesis." Lehninger Principles of Biochemistry, by David L. Nelson et al., Basingstoke, 2017, pp. 824-825.
- ↑ Liu CH, Chang SH, Narko K, Trifan OC, Wu MT, Smith E, Haudenschild C, Lane TF, Hla T. Overexpression of cyclooxygenase-2 is sufficient to induce tumorigenesis in transgenic mice. J Biol Chem. 2001 May 25;276(21):18563-9. doi: 10.1074/jbc.M010787200. Epub 2001 , Mar 7. PMID:11278747 doi:http://dx.doi.org/10.1074/jbc.M010787200
- ↑ 7.0 7.1 7.2 7.3 Vecchio AJ, Simmons DM, Malkowski MG. Structural basis of fatty acid substrate binding to cyclooxygenase-2. J Biol Chem. 2010 Jul 16;285(29):22152-63. Epub 2010 May 12. PMID:20463020 doi:10.1074/jbc.M110.119867
- ↑ 8.0 8.1 Chan CC, Boyce S, Brideau C, Charleson S, Cromlish W, Ethier D, Evans J, Ford-Hutchinson AW, Forrest MJ, Gauthier JY, Gordon R, Gresser M, Guay J, Kargman S, Kennedy B, Leblanc Y, Leger S, Mancini J, O'Neill GP, Ouellet M, Patrick D, Percival MD, Perrier H, Prasit P, Rodger I, et al.. Rofecoxib [Vioxx, MK-0966; 4-(4'-methylsulfonylphenyl)-3-phenyl-2-(5H)-furanone]: a potent and orally active cyclooxygenase-2 inhibitor. Pharmacological and biochemical profiles. J Pharmacol Exp Ther. 1999 Aug;290(2):551-60. PMID:10411562