Sandbox Reserved 1472
From Proteopedia
Ť
| This Sandbox is Reserved from November 5 2018 through January 1, 2019 for use in the course "CHEM 4923: Senior Project taught by Christina R. Bourne at the University of Oklahoma, Norman, USA. This reservation includes Sandbox Reserved 1471 through Sandbox Reserved 1478. |
To get started:
More help: Help:Editing |
This page is reserved for Tim
Contents |
Hsp90-Cdc37-Cdk4 Complex
|
Structural highlights
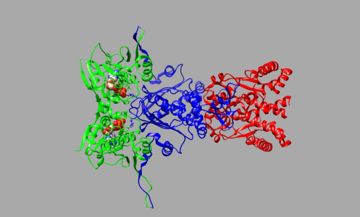
The Hsp90-Cdc37-Cdk4 complex is made up of the Heat Shock Protein 90 chaperone molecule, Cell Division Cycle 37 co-chaperone molecule, and the Cyclin-dependent 4 Kinase client molecule. In the cryo-electron microscopy structure of the Hsp90-Cdc37-Cdk4 complex the β4-β5 sheet of Cdk4 is unfolded which separates it into two lobes, Cdc37 wedges itself between these lobes, and Hsp90 clamps around the β5 sheet of Cdk4[1]. While Cdk4 as the client protein of this complex is important because its proper functioning is the intended goal of the complex, the is the key factor in the process. The main structure of Hsp90 can be broken down into three domains; N-terminal domain (NTD), Middle domain (MD), and the C-terminal domain (CTD), see Figure 1 (NTD-Green, MD-Blue, CTD-Red). The NTD is the site where ATP binds closing the "clamp", the MD is primarily the site of client binding, and the CTD is responsible for dimerization of the promoters that forms the biological unit homodimer[2]. Before the full complex is formed Cdc37 will bind with Cdk4 forming a (Cdc37-Pink, Cdk4-Yellow). Cdc37 as in Hsp90 can be broken down into a CTD, MD, and NTD. In the full ternary complex Cdc37's MD binds to the ATP lid-segment, a group of residues that when ATP binds to Hsp90 they fold over to trap the nucleotides. Cdc37's NTD interacts mostly with the client protein and it's CTD has binding interactions with the Hsp90 homodimer[3]. Cdk4 structure is more simple than Hsp90 and Cdc37 only consisting of an NTD and a CTD. Molecular dynamic simulations have shown that when complexed with cyclin-D3 in an inactive conformation considerable flexibility was noted in its N-lobe regions notably at its αC-helix, αC-β4 loop, and β4-β5 sheet[4]. The is an intricate multicomponent system made of a generalized clamping mechanism, a narrowing adaptive recruiter/tuner, and dynamic protein client molecule.
Function
Hsp90 is a 90-kDa protein that acts as a molecular chaperone responsible for protection and proper functioning in a variety of client proteins the majority of which are involved in signal transduction. It belongs to a group of proteins called heat shock proteins. Heat shock proteins are named for the response that organisms exhibit due to stresses at a cellular level such as elevated temperatures beyond the normal existing environment cells usually cope with. A major feature of this response is an alteration of an organism’s gene expression through increased heat shock protein production[2]. Cdc37 is one the the main co-chaperones of Hsp90, it recruits kinase proteins for late folding and activation by binding to the kinase and delivering it to Hsp90. Cdk4 is a part of a kinase complex that is needed for cell cycle G1 phase progression. For the activation of Cdk4 there must be proper folding, binding of the regulator cyclin D, ATP binding, a positioning of the active site near the ATP binding site and phosphorylation of the amino acid residue T172. Hsp90 and the co-chaperone Cdc37 play a crucial role in the late folding process of Cdk4 needed for its activation of which is critical for cell development[5]. When reading the current reseach material about Hsp90 it is generally stated that Hsp90 is involved in folding activities of its clients, however the Hsp90 homodimer is a very simple physical clamping mechanism and there is little detail currently in what ways Hsp90 is folding client proteins. Another major manner in which Hsp90 may be playing in its role as a chaperone for clients is by simply staging them to be quickly activated when and where needed. Cdk4 is bound to the Hsp90-Cdc37 complex in a way that renders it completely inactive and it is released in active form at the end of the G1 cell phase when the concentration of cyclin D has increased. Cdk4 then travels to the nucleus where it is involved in phosphorylating retinoblastoma gene products[5]. Hsp90 may be staging Cdk4 in the vicinity of where it needs to be and releasing it with temporal management allowing Cdk4 to perform its function with efficiency. A third chaperone duty Hsp90 may be performing for clients is to protect them from degredative processes such as being targeted by ubiquitin[4]. This may be especially the case with a client like Cdk4 that does show a tendency to be unstable at least in part, with its instability being inherent to its functioning as a dynamic triggering molecular apparatus. In fact is has been observed that blocking kinase clients of Hsp90 from interacting with Cdc37 promoted their degradation[3].
Mechanics & Energetics
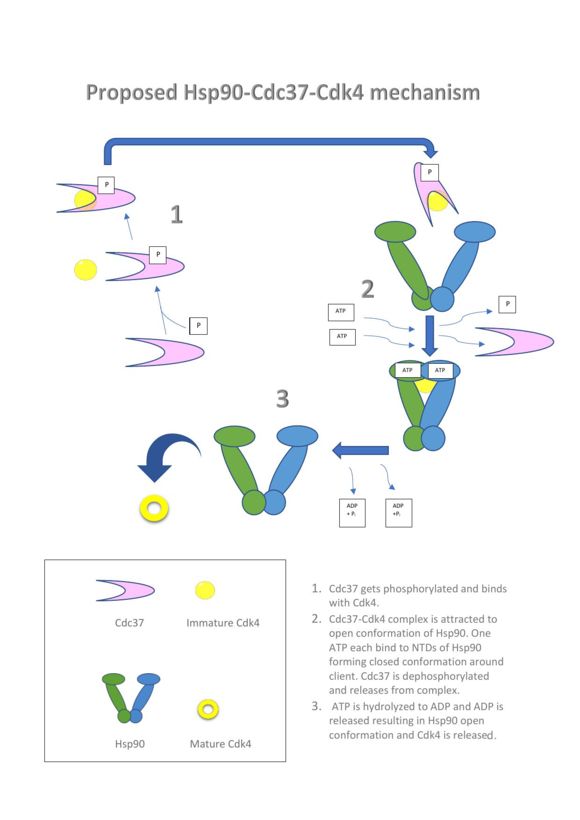
Hsp90 and the co-chaperone Cdc37 are responsible for about 60% of kinases to achieve an active state[1]. One of the questions that researchers have had is why some kinases are clients of Hsp90 and Cdc37 and not others. There is evidence Cdc37 works by recognizing conformational instability of kinase clients and changing their folding landscapes as it binds to the client and recruits it to Hsp90[4]. In this way Cdc37 would be selecting kinase clients for Hsp90, where less thermodynamically stable clients would be delivered to Hsp90 and more stable clients would not. The open and closed conformations of Hsp90 are driven by the binding and release of adenosine triphosphate (ATP) in the N-terminal domain (NTD), where the closed state has ATP bound and the open state does not. Before Hsp90 will interact with Cdc37 and Cdk4 they will first interact with each other, see proposed Hsp90-Cdc37-Cdk4 mechanism Figure 2. Evidence points to the NTD of Cdc37 being the region that recognizes both client and non-client kinases. When the NTD of Cdc37 was removed it was found to fail in interacting with bRaf, another known kinase client of the Hsp90-Cd37 system[6]. The CTD of Cdc37 has been found to function in providing additional protection when the Cdk4 client is in a partially unfolded state during the binding process[4] The action that initiates Cdc37 interaction with kinases such as Cdk4 is its phosphorylation by Casein Kinase 2. Then once Cdc37 has interacted with Cdk4, opening it by a separation of it's NTD and CTD followed by binding of the two proteins together they will come into contact with an open conformation Hsp90 homodimer. ATP will then bind to the NTDs of Hsp90 and cause it to close trapping the partially opened Cdk4 molecule client.Cdc37 is dephosphorylated by Protein Phosphatase 5 allowing its release. Hsp90 will open upon ATP hydrolysis resulting in the release of the client protein[1]. When Hsp90 is in the chaperone-client complex it becomes more stable than when it is by its self. Hsp90 MD residues in its core as well as residues 398-453 that from a three-helix bundle both exhibit reduced thermal fluctuations as new intra- and intermolecular interactions are formed when in the chaperone-client complex. The residues 513-519 that are interfacial MD-CTD residues also show an increase in stability through reduced thermal fluctuations as does helix 15 in the CTD. While the MDs and CTDs of the Hsp90 homodimer are stabilized in the chaperone-client complex the NTDs undergo and increase in flexibility. When transitioning from an unbound state to the chaperone-client complex Hsp90 experiences a relocation of major hinge clusters shifting from the NTD and NTD-MD regions to its MD-CTD regions as this happens the residues remaining in the NTD become less stabilized[4]. The client binding surfaces of Hsp90 range over a large area from its NTDs to its MDs and this is indicative of Hsp90 forming lots of low-energy binding patches with clients[5]. It must be remembered that Cdk4 is only one of many Hsp90 client proteins and Cdc37 only one of a number of Hsp90 co-chaperones. It can be inferred that Hsp90 uses the large surface area generalized binding interfaces as an effective strategy to deal with multiple clients. Also the use of co-chaperones is another way in which Hsp90 can fine tune its interactions with the multiple clients it must interact and manipulate. Another heat shock protein named HSP70 is often involved with Hsp90 chaperoning systems. When these chaperones are involved in interacting with the same client, Hsp90 interaction will follow Hsp70 client interaction. Hsp70 identifies and is able to help with the proper folding of hydrophobic areas of proteins. The interfaces of Hsp90 are less hydrophobic with charged residues being more prevalent. The binding interfaces for Cdk4 and Tau a protein that functions in microtubule construction and stabilization interact with Hsp90 residues that have a net positive charge. However it is possible for other clients to bind to Hsp90 where it has a net negative charge such as glucocorticoid receptor (GR) a steroid hormone receptor that regulates development, metabolism, and immunes responses. Cdk4 contrast with both Tau and GR in that its binding interfaces are actually much more hydrophobic in nature that the other two[5]. Overall the binding complex of Hsp90-Cdc37-Cdk4 causes global conformational rigidity in the binding areas of Hsp90, and at the same time Cdk4 is rendered less stable by having its domains split apart while complexed. This leads to a reversible entropy transfer where the chaperone flexible regions are ordered while allowing the client to be staged within the complex in a less stable more dynamic state[4].
Relevance (Disease)
Disease causes cellular stress in organisms and because heat shock proteins function to protect proteins from stressed conditions they have relevance in many pathological processes like cancer, neurodegenerative disease, infectious agents and more[2].Because Hsp90 works so flexibly to stabilize proteins it becomes a problem by helping oncogenic proteins function. However this is also a potential tool in fighting cancer by Hsp90 inhibition. Hsp90 has a high basal expression compared to most other heat shock proteins. If the organism is not under environmental stress Hsp90 may not be needed at high levels for homeostasis, however cancer cells are highly reliant on it. The largest interest in the recent study of Hsp90 is in its use as a cancer treatment.The two main natural inhibitors that can be used to inhibit Hsp90 are radicicol and geldanamycin. These inhibitors work by binding to Hsp90 where normally ATP would. These natural inhibitors have been studied in vitro and in vivo and have shown to affect cancer proliferation but they both have problems in regards to instability and toxicity[2]. One method deal with the issue of toxicity and instability of these natural inhibitors is to alter them. This has been done with geldanamycin by switching its C17 with an allylamino group, this results in 17-AAG (17-allyl-17-demethoxygeldamycin). This has helped with the toxicity issue and 17-AAG has been used in phase I/II clinical trials[2]. Another method that could be used to inhibit the Hsp90 system to attack cancer is to target other parts of the system rather than the ATP binding in the NTD of Hsp90. For instance, the interaction between Hsp90 and one of its main co-chaperones could be disrupted. This could lead to a narrowed impact on Hsp90 function and be less harmful to the body’s healthy cells. Cdc37 is an excellent example of a co-chaperone whose interaction with Hsp90 could be targeted and this would allow client proteins other than Hsp90 dependent kinases to still function properly. Many of the kinase clients of Hsp90-Cdc37 are involved in the regulation of cellular responses and development and the proliferation of cancer cells are especially susceptible by their disruption[7]. Three ways in which the Hsp90-Cdc37-client protein complex functioning could be interfered with are; targeting Cdc37, the interaction between Cdc37 and the client protein, or the interaction between Hsp90 and Cdc37. Cdc37 has an increased level in growing tissues and shows high expression in some tumor cells, it could be possible to limit Cdc37 through gene silencing techniques. Targeting Cdc37 client interaction could be done through disrupting the phosphorylation of Cdc37 that needs to happen to initiate its initial interaction with clients. There are already several disruptor molecules of Hsp90-Cdc37 interaction that have been identified or synthesized with multiple mechanisms of blocking, some like Cdc37-derived Peptide Pep-1 simply binds to Hsp90 acting competitively against Cdc37 binding[7]. Hsp90 has the potential to serve as an effective means in fighting cancer. Targeting the Hsp90-Cdc37 chaperone system could be used make Hsp90 a more precise weapon in this battle by limiting toxicity issues in normal cells.
References
- ↑ 1.0 1.1 1.2 Verba KA, Wang RY, Arakawa A, Liu Y, Shirouzu M, Yokoyama S, Agard DA. Atomic structure of Hsp90-Cdc37-Cdk4 reveals that Hsp90 traps and stabilizes an unfolded kinase. Science. 2016 Jun 24;352(6293):1542-7. doi: 10.1126/science.aaf5023. PMID:27339980 doi:http://dx.doi.org/10.1126/science.aaf5023
- ↑ 2.0 2.1 2.2 2.3 2.4 Hoter A, El-Sabban ME, Naim HY. The HSP90 Family: Structure, Regulation, Function, and Implications in Health and Disease. Int J Mol Sci. 2018 Aug 29;19(9). pii: ijms19092560. doi: 10.3390/ijms19092560. PMID:30158430 doi:http://dx.doi.org/10.3390/ijms19092560
- ↑ 3.0 3.1 Pearl LH. Review: The HSP90 molecular chaperone-an enigmatic ATPase. Biopolymers. 2016 Aug;105(8):594-607. doi: 10.1002/bip.22835. PMID:26991466 doi:http://dx.doi.org/10.1002/bip.22835
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 Czemeres J, Buse K, Verkhivker GM. Atomistic simulations and network-based modeling of the Hsp90-Cdc37 chaperone binding with Cdk4 client protein: A mechanism of chaperoning kinase clients by exploiting weak spots of intrinsically dynamic kinase domains. PLoS One. 2017 Dec 21;12(12):e0190267. doi: 10.1371/journal.pone.0190267., eCollection 2017. PMID:29267381 doi:http://dx.doi.org/10.1371/journal.pone.0190267
- ↑ 5.0 5.1 5.2 5.3 Radli M, Rudiger SGD. Dancing with the Diva: Hsp90-Client Interactions. J Mol Biol. 2018 Sep 14;430(18 Pt B):3029-3040. doi: 10.1016/j.jmb.2018.05.026., Epub 2018 May 18. PMID:29782836 doi:http://dx.doi.org/10.1016/j.jmb.2018.05.026
- ↑ Keramisanou D, Aboalroub A, Zhang Z, Liu W, Marshall D, Diviney A, Larsen RW, Landgraf R, Gelis I. Molecular Mechanism of Protein Kinase Recognition and Sorting by the Hsp90 Kinome-Specific Cochaperone Cdc37. Mol Cell. 2016 Apr 21;62(2):260-71. doi: 10.1016/j.molcel.2016.04.005. PMID:27105117 doi:http://dx.doi.org/10.1016/j.molcel.2016.04.005
- ↑ 7.0 7.1 Li T, Jiang HL, Tong YG, Lu JJ. Targeting the Hsp90-Cdc37-client protein interaction to disrupt Hsp90 chaperone machinery. J Hematol Oncol. 2018 Apr 27;11(1):59. doi: 10.1186/s13045-018-0602-8. PMID:29699578 doi:http://dx.doi.org/10.1186/s13045-018-0602-8