Structure
The structure at right can be organized into both broad regions and more defined domains. There are three main regions: N-terminal region, catalytic region, and C-terminal glucan binding region.[1] The domains are more complex. There are five total domains; however, only four are visible in this crystal structure. Each domain consists of an N-terminal and C-terminal portion of the polypeptide chain, except Domain C, which is located at the bottom of the enzyme.[1] Following the polypeptide chain from N-terminus to C-terminus would pass through the domains in the following order: V, IV, B, A, C, A, B, IV, V.[2] A high degree of structural similarity exists within the GH70 family enzymes.[2]
Catalytic Domain
Domain A is regarded as the catalytic domain.[1] Within it, exists a highly conserved , which includes two aspartic acid residues and a general acid/base glutamate.[1] A calcium ion is also bound to help stabilize the formation of the catalytic domain.[1]
Supersecondary Structure
This enzyme features two well-known motifs. Domain A consists of a TIM barrel.[1] It is composed of a ring of beta strands surrounded by a ring of alpha helices. Domain C contains a greek key motif, which is four antiparallel beta strands that form a sheet.[2]
Function and Energetics
The major functions of this enzyme include synthesis of glucan, a glucosylated acceptor molecule, or release of free glucose.
[3] 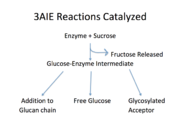
Reactions catalyzed by 3aie, mutansucrase of S. mutans. Image adapted from Monchois, et al.
As shown in Figure 1, all of the products are released from the same active site and from a glucosyl-enzyme intermediate.
[3] The sole, natural substrate for this enzyme is sucrose.
[4] No cofactors or energy-carriers are required for these reactions.
[3] The energy required for bond formation is solely provided by the energy released from sucrose hydrolysis.
[3]This particular glucansucrase is a mutansucrase, meaning it catalyzes the formation of α(1,3) linked glucose moieties.
[1] The latest research proposes that the glucose moieties are most likely added to the non-reducing end of the glucan chain.
[5] Binding of a glucan chain to the active site may cause a conformational change within the enzyme that favors elongation of the glucan chain instead of the formation of the other products.
[3] Acceptor molecules include maltose and isomaltose.
[2] Free glucose may be released upon hydrolysis of the glucose-enzyme intermediate.
[2]
Disease and Relevance
As mentioned previously, one of the products of this enzyme is glucan. This glucose polymer comprises the biofilm, which plays a particularly important role in the formation of cavities.[4] The sticky glucan helps to adhere S. mutans and other bacteria to the tooth surface, where acids can penetrate and degrade the tooth due to this localization.[4] In fact, Hamada, et al state that the primary product of this enzyme comes from the fermentation of sucrose to yield lactic acid. Based on the activity of this enzyme, it appears to be a good candidate for inhibition.
Using the crystal structure of this enzyme bound to known inhibitor acarbose (PDB entry: 3aic), Zhang et al were able to create several competitive inhibitors.[6] Two of the inhibitors were found to be highly selective for this enzyme and reduced glucan production to 15% of the wild-type activity in rats.
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 Ito K, Ito S, Shimamura T, Weyand S, Kawarasaki Y, Misaka T, Abe K, Kobayashi T, Cameron AD, Iwata S. Crystal Structure of Glucansucrase from the Dental Caries Pathogen Streptococcus mutans. J Mol Biol. 2011 Feb 25. PMID:21354427 doi:10.1016/j.jmb.2011.02.028
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 Leemhuis H, Pijning T, Dobruchowska JM, van Leeuwen SS, Kralj S, Dijkstra BW, Dijkhuizen L. Glucansucrases: three-dimensional structures, reactions, mechanism, alpha-glucan analysis and their implications in biotechnology and food applications. J Biotechnol. 2013 Jan 20;163(2):250-72. doi: 10.1016/j.jbiotec.2012.06.037. Epub, 2012 Jul 10. PMID:22796091 doi:http://dx.doi.org/10.1016/j.jbiotec.2012.06.037
- ↑ 3.0 3.1 3.2 3.3 3.4 Monchois V, Willemot RM, Monsan P. Glucansucrases: mechanism of action and structure-function relationships. FEMS Microbiol Rev. 1999 Apr;23(2):131-51. doi:, 10.1111/j.1574-6976.1999.tb00394.x. PMID:10234842 doi:http://dx.doi.org/10.1111/j.1574-6976.1999.tb00394.x
- ↑ 4.0 4.1 4.2 Hamada S, Slade HD. Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiol Rev. 1980 Jun;44(2):331-84. PMID:6446023
- ↑ Moulis C, Joucla G, Harrison D, Fabre E, Potocki-Veronese G, Monsan P, Remaud-Simeon M. Understanding the polymerization mechanism of glycoside-hydrolase family 70 glucansucrases. J Biol Chem. 2006 Oct 20;281(42):31254-67. doi: 10.1074/jbc.M604850200. Epub 2006, Jul 24. PMID:16864576 doi:http://dx.doi.org/10.1074/jbc.M604850200
- ↑ Zhang Q, Nijampatnam B, Hua Z, Nguyen T, Zou J, Cai X, Michalek SM, Velu SE, Wu H. Structure-Based Discovery of Small Molecule Inhibitors of Cariogenic Virulence. Sci Rep. 2017 Jul 20;7(1):5974. doi: 10.1038/s41598-017-06168-1. PMID:28729722 doi:http://dx.doi.org/10.1038/s41598-017-06168-1

