Sandbox Reserved 1506
From Proteopedia
|
Contents |
Function
Serotonin N-acetyltransferase is also named arylalkylamine-N-acetyltransferase (ANAAT). AANAT is a member of a large superfamily of proteins, the GCN-5-related N-acetyltransferase (GNAT) superfamily, also know as the motif A/B superfamily. Enzymes from this superfamily catalyse the acetylation of all sorts of residues.
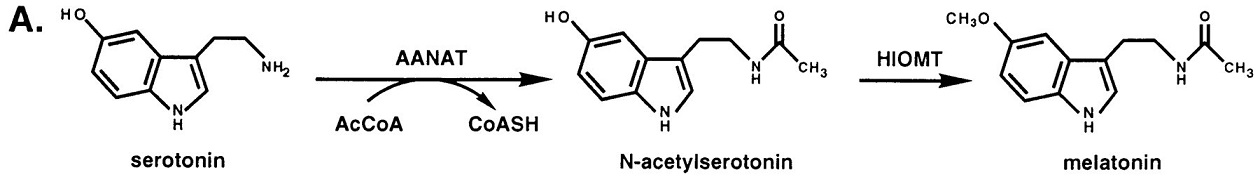
The role of ANAAT is to catalyze the acetylation of the amine group held by serotonin, an intermediate in melatonin synthesis. ANAAT is the penultimate enzyme in the melatonin pathway, as it is shown on the picture below. [1].
It is synthesised into the pineal gland.
Relevance
Circulating melatonin plays a major role in the circadian rythm. Day/night differences in circulating melatonin levels provide a hormonal analog signal of environmental lighting, which is used in a variety of ways to optimize circadian and circannual rhythms in physiology[2].
The amount of circulating melatonin is correlated with light exposure of the eyes and with ANAAT activity, as the activity of the enzyme varies in parallel with melatonin amount. For example, in some species the night/day differences in melatonin, AANAT activity, and protein are 10- to 100-fold [3][4].
In that respect; defects in ANAAT regulation could be responsible for sleep troubles, such as insomnia or narcolepsy,[5] but also serotonin-related deseases such as depression [6].
Structure
|
General description
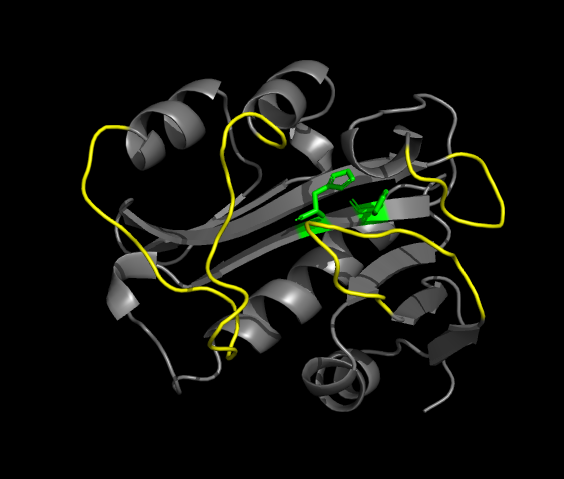
is a 384-residues globular protein consisting of an 8-stranded β sheet with five α helices. There is a conserved motif in the center of the β sheet which forms the binding site for acetyl-CoA, the acetyl source. The β sheet is highly twisted and displays a bulge on its middle strand ( in green on the figure). A long α helix lies on the concave surface of the β sheet, with its axis approximately aligned with those of the strands. Two other helices wraps around the convexe surface of the sheet, perpendicular to it, like the staves of a barrel.
The Acetyl-CoA binding site
The pantetheine group of Ac-CoA binds in a cleft formed at the site where two parallel β strands come apart. It is held in place by several H-bonds. The turn joining a strand of the β sheet and the long α helix form a positively charged pocket to welcome the pyrophosphate group.
The active site
The folding of the active site is similar to the one of other members of the GNAT superfamily. However, the active site is deeply burried into the protein, which is rare in the GNAT family. The access to this site is warranted for serotonin by and hydrophobic funnel formed by three polypeptide loops which converge above the acCoA binding site.
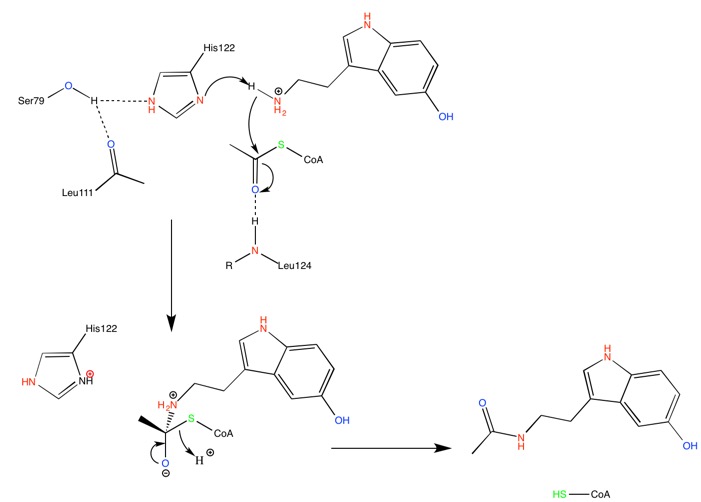
At the bottom of the funnel, in the active site, are located two conserved histidines which suggest a catalytic mechanism for acetylation involving imidazole groups acting as general acid/base catalysts [7]. The supposed calytic role of these histidines, H120 and H122, is illustrated by the following mechanism.
His-122 is hold in place through H-bond interaction with a serine residue, Ser-97, suggesting this particular histidine to be the main catalytic residue. Mutations of either of these histidine residues result in a complete loss of catalytic function.
Right after catalytic histidine H122, Ala-123 is responsible for a bulge in the β sheet. It is an important feature of the active site, and it also accentuate the twist of the β sheet.
Access to the active site
The entrance of the funnel is surrounded by polar residues but then then many hydrophobic residues are lining the sides of the funnel. It makes of size a dominant factor for the access to the active site, as only small-sized molecule can reach the acetyl group of acCoA. Hydrophobic nature of the funnel is another selective critirion.[8] Thus no specific protein-substrate interaction has been idientified, because several different substrates than serotonin, such as phenylethylamine, can access the active site and be acetylated.
[9].
The fact that active site is located at the end of a constricted funnel explains well why the rate-limiting step in catalysis is the diffusional realease of the product.[10]
Regulation
The regulation of the AANAT is managed with cAMP-dependant protein kinase phosphorylation [11][12]: firstly, the cAMP controls the AANAT presence through inhibition of proteasomal proteolysis. The variations of circulating melatonin are too important for the gene-regulating way to be considered. cAMP amount in pineal gland is controled by a photoneuronal system including the eyes and the suprachiasmatic nucleus ( the site of the circadian clock ) for mammals, or by light acting on pinealocytes for lower vertebrates.
Furthermore, the cAMP increases the AANAT activity without increasing the AANAT mRNA concentration or the AANAT concentration.
cAMP selectively enhances the intracellular acetylation of the arylalkylamine, regulating it.
There is a 2-tiered system of regulation depending on the concentration of cAMP, involving two activity levels:
- A low cAMP concentration range : AANAT is dual-phosphorylated, on T31 and S205, and display low efficiency.
- A high cAMP concentration range : AANAT is unphosphorylated, and is efficient.
Phosporylation of these two sites promotes the binding of a dimeric protein, 14-3-3. This protein is responsible for the activation of ANAAT by promoting access of the enzyme's substrates ( serotonin ) and prevent ANAAT from proteosomal proteolysis. It is interesting to note that phosporylation of these two sites is essential for the activation of the enzyme, and S205-phosporylation only is responsible for a dramatical inhibition of ANAAT activity.
This is why ANAAT activity, and consequently circulating melatonin amount are linked with light exposure, and form a day/night cycle.
- ↑ Hickman, A. B; Klein, D. C; Dyda, F. Melatonin Biosynthesis: The Structure of Serotonin N-Acetyltransferase at 2.5A Resolution Suggests a Catalytic Mechanism. Mol. Cell. 1999, 3-1, 23-32
- ↑ Arendt, J. (1995) Melatonin and the Mammalian Pineal Gland (Chapman & Hall, London), pp. 201–285.
- ↑ Klein, D.C., and Weller, J.L. (1972). Rapid light induced decrease in pineal serotonin N-acetyltransferase activity. Science 177, 532–533.
- ↑ Gastel, J.A., Roseboom, P.H., Rinaldi, P.A., Weller, J.L., and Klein,D.C. (1998). Melatonin production: proteasomal proteolysis in serotonin N-acetyltransferase regulation. Science 279, 1358–1360
- ↑ Rati Srinivasan, Guy Gechtman, Abe Bayer. What is the Molecular Basis of Sleep Regulation, https://sites.tufts.edu/sleep/
- ↑ Hickman, A. B; Klein, D. C; Dyda, F. Melatonin Biosynthesis: The Structure of Serotonin N-Acetyltransferase at 2.5A Resolution Suggests a Catalytic Mechanism. Mol. Cell. 1999, 3-1, 23-32
- ↑ Hickman, A. B; Klein, D. C; Dyda, F. Melatonin Biosynthesis: The Structure of Serotonin N-Acetyltransferase at 2.5A Resolution Suggests a Catalytic Mechanism. Mol. Cell. 1999, 3-1, 23-32.
- ↑ Coon, S.L., Roseboom, P.H., Baler, R., Weller, J.L., Namboodiri, M.A.A., Koonin, E.V., and Klein, D.C. (1995). Pineal serotonin N-acetyltransferase: expression cloning and molecular analysis.Science 270, 1681–1683.
- ↑ Deguchi, T. (1975). Characteristics of serotonin-acetyl coenzyme A N-acetyltransferase in pineal gland of rat. J. Neurochem. 24, 1083-1085.
- ↑ Khalil, E.M., De Angelis, J., and Cole, P.A. (1998). Indoleamine analogs as probes of the substrate specificity and catalytic mechanism of serotonin-N-acetyltransferase. J. Biol. Chem. 273, 30321–30327.
- ↑ The cAMP regulation of Arylalkylamine-N-acetyltransferase - Steven L. Coon, Joan L. Weller, Horst-W. Korf, M. A. A. Namboodiri, Mark Rollagi, and David C. Klein - The Journal of Biological Chemistery vol. 275 n°26, April 17, 2001.
- ↑ Melatonin synthesis: 14-3-3-dependent activation and inhibition of arylalkylamine N -acetyltransferase mediated by phosphoserine-205 - Surajit Ganguly, Joan L. Weller, Anthony Ho, Philippe Chemineau, Benoit Malpaux, and David C. Klein - PNAS, January 25, 2005.