Sandbox Reserved 162
From Proteopedia
|
Contents |
Background
Human Interleukin-6 (IL-6) is a immune protein categorized under hematopoietins much like Interleukin-11, Leukemia Inhibitory Factor (LIF), Oncostatin M (OM), Ciliary Neurotrophic Factor (CNTF), and Cardiotrophin-1 (CT-1) [1]. IL-6 is produced by various types of lymphoid and non-lymphoid cells, such as T cells, B cells, monocytes, fibroblasts, kerationcytes, endothelial cells, mesangium cells, and several tumor cells [2]. Since its discovery, it has been known by many names including: interferon-ß2 (IFN-ß2)[3][4], 26-kD protein[5][6], B-cell stimulatory factor-2 (BSF-2)[7], hepatocyte stimulating factor (HSF)[8], cytotoxic T-cell differentiation factor (CDF)[9], and hybridoma/plasmacytoma growth factor (HPGF/HGF)[10][11][12]. It wasn't until December of 1988 that is was givin the final moniker of IL-6 (PDB: 1IL6)[1].
Structure
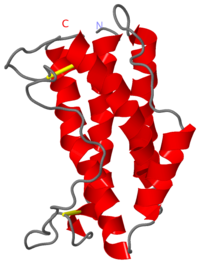
This structure of Interleukin-6 was crystallized at 1.9Å. IL-6 is a monomer of 185 amino acids coded by a gene found at locus 7p21 which codes for four introns and five exons. It contains , four of which constitute a classical four-helix bundle with the fifth helix located in the C-D loop. The four helices that form the four-helix bundle are arranged so that two helices (A & B) run in the same direction and two (C & D) in opposing directions. The N-terminal 18 amino acids of IL-6 are not visible in electron density maps and consequently have not been modeled. The first long helix extends from Ser21 to Ala45 and is connected to a parallel helix (B) by a 25 amino acid loop. This helix extends from Glu80 to Gln102. A short connection formed from Asn103 to Ser108 joins this helix to the next helix . This third helix extends from Glu109 to Lys129 and is followed by another amino acid loop which includes a of three turns (Pro141-Gln152). The last helix extends from Gln156 to Arg182[13].
Functions
IL-6 is a potent polyfunctional cytokine that plays a vital role in host defense. It is released in response to infection, burns, trauma, and neoplasia[14]. Its polyfunctional role is demonstrated by the ability to induce responses in the immune system, hematopoietic system, and in acute-phase reactions. In the immune system, IL-6 is an essential factor for antibody production in B cells and acts as a potent growth factor for myeloma cells. IL-6 also acts on the final maturation stage of activated B cells, can be effective on resting T cells, and induces differentiation of cytotoxic T cells. In the hematopoietic system, IL-6 (in combination with Interleukin-3) activates and accelarates the growth of hematopoietic stem cells during the G0 phase causing them to enter the G1 phase of the cell cycle. With respect to acute-phase reactions, IL-6 plays a key role in enhancing the innate immune system by inducing the expression of mRNA's for proteins associated with acute-phase reactions. These proteins are resleased into blood plasma by liver cells and protect against tissue damage. Moreover, IL-6 is thought to cross the blood brain barrier and induce synthesis of Prostaglandin E2 (PGE2) in the hypothalmus which changes the body's temperature setpoint resulting in fever (Citation).
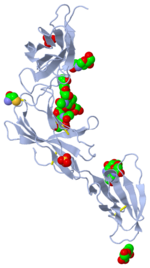
IL-6 Receptor Complex
|
The IL-6 receptor (IL-6R) is located on the surface of resting normal T-cells, activated normal B-cells, myeloid cell lines, hepatoma cell lines, and myeloma cell lines (Citation). The IL-6R complex is composed of two molecules of IL-6 and two molecules of IL-6R, along with the signaling molecule gp130 (Citation).
Clinical Applications
As IL-6 plays many parts in the body, research is continually discovering new roles in which it plays. Disregulated production of IL6 and its receptor are implicated in the pathogenesis of many diseases including diabetes, atherosclerosis, depression, Alzheimer's Disease, systemic lupus erythematosus, prostate cancer, rheumatoid arthritis, and multiple myeloma. A good correlation has been found between levels of IL-6 and bone loss in patients with Paget's disease and multiple myeloma. In those patients, higher levels of IL-6 correlated to higher rates of bone loss. Additionally, IL-6 levels decreased with increasing levels of estrogen. These findings strongly suggest that IL-6 plays a key roll in post-menopausal bone loss (Citation). Early research, where bone marrow cells used in transplants were precultured with IL-6 and IL-3, showed an increase in post-transplant cell survival rate from 20% to 90% (Citation). If this culture system can be expanded for human stem cells, it may be used in bone marrow tansplantation (Citation). Other research has indicated that IL-6 is an important factor in malignant cells of Kaposis sarcoma and multiple myeloma (Citation).
Additional Resources
References
- ↑ 1.0 1.1 Sehgal P. et al, "Interleukin-6-type cytokines." Annals of the New York Academy of Sciences, 1995, 762
- ↑ Kishimoto, T. "The Biology of Interleukin 6." Am J Hematol, Jul, 1989, 74(1), 1-10.
- ↑ Weissenbach I. et al, "Two interferon mRNAs in human fibroblasts: In vitro translation and Escherichia coli cloning studies." Proc Narl Acad Sci USA, 1980, 777152-7156.
- ↑ Zilberstein et al, "Structure and expression of cDNA and genes for human interferon-beta-2, a distinct species inducible by growth-stimulatory cytokines." EMBO J 1986, 5,2529-2537.
- ↑ Content, J. et al, "Secretory proteins induced in human fibroblasts under conditions used for the production of interferon beta." Pmc Natl Acad Sci USA, 1982, 792768-2772.
- ↑ Haegeman G. et al, "Structural analysis of the sequence coding for an inducible 26-kDa protein in human fibroblasts." Eur J Biochem, 159625-632.
- ↑ Hirano et al, "Purification to homogeneity and characterization of human B-cell differentiation factor (BCDF or BSF-2)." Eur J Immunol, 1986, 181797-1801
- ↑ Gauldie et al, "Interferon P2/B-cell stimulatory factor type 2 shares identity with monocyte-derived hepatocyte-stimulating factor and regulates the major acute phase protein response in liver cells." Proc Natl Acad Sci USA, 1987, 847251-7255
- ↑ Takai et al, "B cell stimulatory factor-2 is involved in the differentiation of cytotoxic T lymphocytes." J Immunol, 1988, 140508-512
- ↑ van Snick J. et al, "Purification and NH2-terminal amino acid sequence of a T cell-derived lymphokine with growth factor activity for B-cell hybridomas." Proc Natl Acad Sci USA, 1986, 83, 9679-9683.
- ↑ Brakenhoff JPJ et al, " Molecular cloning and expression of hybridoma growth factor in Esherichia coli." J Immunol, 1987, 197, 4116-4121
- ↑ Nordan et al, "Purification and NH2-terminal amino acid sequence of a plasmacytoma growth factor derived from the murine macrophage cell line, P388D1." J lmmunol, 1987, 139813-817.
- ↑ Somers W, Stahl M, Seehra J, "1.9Å crystal structure of interleukin 6: implications for a novel mode of receptor dimerization and signaling." EMBO J, 1997, 16(5), 989-997.
- ↑ Janeway, et al. 2001. Immunobiology: The Immune System in Health and Disease. New York(NY): Garland Science.