Introduction
Background
Gamma secretase (GS) is a transmembrane aspartatic protease that catalyzes peptide bond hydrolysis of type I integral membrane proteins such as Notch, amyloid precursor protein (APP), and various other substrates. GS recognizes and catalyzes the cleavage of its substrate into 3 residue segments.[1] Initial APP processing by Beta secretase includes the 48-residue peptide Aβ48 or the 49-residue peptide Aβ49. GS then cleaves these peptides into a variety of peptide fragments separated by 3 residues; Aβ48 is cleaved into Aβ45, Aβ42, and Aβ38; Aβ49 is cleaved into Aβ46, Aβ43, and Aβ40. Aβ products are connected to neurological diseases such as Alzheimer's disease (AD), with varying length peptide products showing different disease symptoms. The connection between GS and AD has made GS a popular drug target. Although various inhibitors of GS have been identified, no inhibitors have been clinically approved for treating AD, as GS is also linked to important neurological functions and inhibition of these GS functions leads to dangerous side effects upon inhibition.[2] Recent cryo-electron microscopy (cryo-EM) structures of gamma secretase have showcased the complex four subunit architecture of gamma secretase, the dynamics required for substrate recognition and cleavage, and the differential binding of gamma secretase to the substrates APP and Notch.[3][4][5][2]
Overall Structure
GS is composed of 20 transmembrane components (TMs) and has four separate protein subunits: Nicastran (NCT), Presenilin (PS1), Anterior Pharynx-defective 1 (APH-1), and Presenilin Enhancer 2 (PEN-2).[3] These subunits are stabilized as functional GS by predominately hydrophobic interactions and four phosphatidylcholines.
has a large extracellular domain and one TM. NCT is important to substrate recognition and binding.
serves as the active site of the protease and contains nine TMs, each varying in length. The site of autocatalytic cleavage is located between of PS1 and a major conformational changes take place in the PS1 subunit upon substrate binding.
serves as a scaffold for anchoring and supporting the flexible conformational changes of PS1.
Activation of the active site is dependent on the binding of . PEN-2 is also important in maturation of the enzyme.[5] Finally, the five reside in the following subunit interfaces between: PS1 and PEN-2, APH-1 and PS1, APH-1 and NCT.
Structural highlights
Substrate Structure
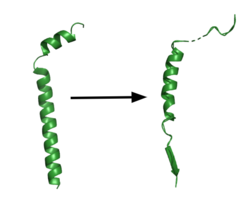
Figure 1. APP fragment conformational change in gamma secretase. APP bound to GS undergoes a conformational change. The free state consists of 2 helices. Once bound to GS, the N-terminal helix unfolds into a coil and the C-terminal helix unwinds into a β-strand. Then the APP β-strand of APP forms a β-sheet interaction with PS1. Cleavage by the protease occurs between the helix and the β-strand.
[2][6] GS has been structurally characterized in the presence of both APP and Notch substrates.[2][5] In each of these structures, the substrate bound in a similar location and underwent a similar structural transition upon binding to the active site of GS. Each substrate is composed of an N-terminal loop and a TM helix. The peptide substrate enters the enzyme by via the lid complex, and once in place, the TM helix of the substrate is anchored by . Upon binding to GS, the N-terminal extracellular helix of the substrate unwinds.[6] The substrate's C-terminal end of the TM helix unwinds into a β-strand (Fig. 1). To differentiate substrates, the β-strand is often the main point of identification for the enzyme. Substrate binding induces a structural change in GS, creating two β-strands that form a β-sheet with the one β-strand of the substrate. This β-sheet is in close proximity with the active site, and guides the process of catalysis.[2]
Lid Complex
The is the first point of entry and recognition for the substrate. The lid is located within the NCT subunit between Asn55 and Asn435. This lobe of NCT is divided into two separate subunits; the large and small lobes with Phe287 from the large lobe acting as a pivot between them. Phe287 is surrounded by of the small subunit. The in the small subunit composes a greasy pocket which provides an environment for easy structural movement. The lid consists of 5 aromatic residues, which are highly involved with stabilizing the closed conformation. This conformation is stabilized by , which interacts with Pro424, Phe448, and the aliphatic side chain of Gln420. Once the substrate binds and the lid is opened, a charged, hydrophilic binding pocket is revealed. The pocket contains . The pocket is further involved with substrate binding and recognition once the lid is removed. The lid complex is relatively far away from the catalytic site of the enzyme in PS1 when inactive. Once a substrate binds, the enzyme undergoes a conformational change in which the rotation of the large lobe in relation to the small lobe reorients the substrate for cleavage, by aligning the pocket in NCT to the active site in PS1.[4]
Active Site
The is located between TM6 and TM7 of the PS1 subunit, which is mainly hydrophilic and disordered. Both TM6 and TM7 contribute an aspartate residue to the active site. These two aspartates, Asp257 and Asp385 are located approximately 10.6 A˚ apart when the enzyme is in the inactive state.[4] Substrate recognition is controlled by the closely spaced PAL sequence of . GS becomes active upon substrate binding, when TM2 and TM6 each rotate about 15 degrees to more closely associate. Two β-strands are induced in PS1, creating an with the β-strand of the substrate.[2] The β-strand of the substrate interacts via main chain H-bonds , stabilizing the active site. hydrogen bond to each other and are located 6–7 Å away from the scissile peptide bond of the substrate, allowing catalysis to occur.[5] GS cleaves in 3 residue segments which is driven by the presence of three amino acid binding pockets in the active site.[1]
In APP, the cleavage site is between the helix and the C-terminal β-strand.[2] GS can cleave via different pathways, depending on its starting point, but the 2 most commonly used pathways produce Aβ48 and Aβ49.[1]. Cleavage between yields Aβ49. Tripeptide substrate cleavage starting between results in Aβ48. The accumulation of these Aβ peptides has strong implications in Alzheimer's disease.[2]
Relevance
GS is connected with the development of AD in humans. Aβ fragment build up leads to amyloidplaques in the brain.[7] Plaques in the brain cause severe neural dysfunction over time.
Mutations in GS are also connected with AD. Over 200 GS mutations have been linked to causing AD. These mutations target "hot spots" on the enzyme and seem to have a preponderance at the binding interface between . The vast majority of these mutations are clustered in regions surrounding the C-terminal half of the . Mutations at these locations affect the integrity of APP recruitment and catalysis, implicating a role in the development of Aβ plaques that impair neural function.[2]
Inhibition of GS could be a potential AD treatment, but this would require targeting only APP cleavage over other GS substrates. APP cleavage leads to products such as Aβ42 and Aβ43,[5] which are prone to aggregation and formation of Aβ plaques. Increased product peptide length contributes to aggregations, and many of the mutations within result in elevated ratios of Aβ42 to the shorter Aβ40.[4] The differential binding of APP and Notch to GS provides a starting point for differentiation but will require further follow-up studies to confirm that the structural differences observed are biologically relevant. Currently, to combat this complex situation, differences in binding between different substrates are being utilized to create drugs that selectively inhibit APP binding with GS, and possibly create a more ideal target for AD treatment.[2]

