This is a default text for your page '. Click above on edit this page' to modify. Be careful with the < and > signs.
You may include any references to papers as in: the use of JSmol in Proteopedia [1] or to the article describing Jmol [2] to the rescue.
Function of your Protein
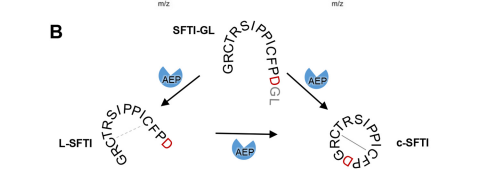
My protein, legumain isoform beta (proAtLEGb), plays a very important role in the plant Arabidopsis thaliana. The specific function of the protein is important for the processing and maturation of the seed storage proteins within storage vacuoles and the programmed cell death and may functionally substitute for the caspases (not found in plants). Our protein is an enzyme and known substrates for it include the pro12Sglobulin and pro2S albumin proteins. The formation of the product is formed from cleavage at the active site by a special activation peptide (AP). The product then takes a cyclic formation after cleavage. More about the active site will be discussed under the amino acid section.
Biological relevance and broader implications
This protein is important for this organism because it deals with seed maturation and how the plant eventually dies.
There really aren't any abnormal events associated with the protein that is known.
Our protein is the first example of a plant legumain capable of linking free termini, which is important because the discovery of these isoform-specific differences will allow us to identify and rationally design efficient ligases with application in biotechnology and drug development.
Important amino acids
To dig deeper and better understand the protein, you can see where the . However, these ligands aren't entirely that important to the active site. The ligands shown are there to help stabilize the structure. The actual important enzyme of the active site is actually the activation peptide(AP). This AP 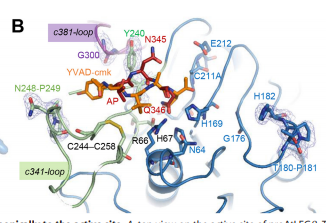 is made up of amino acid residues of ASN345 (asparagine), GLN346 (glutamine), ARG347 (arginine), which form and where the cleavage happens. This cleavage
is made up of amino acid residues of ASN345 (asparagine), GLN346 (glutamine), ARG347 (arginine), which form and where the cleavage happens. This cleavage  basically splits the sequence in half of prime and nonprime on either side of the split. Then, the split sequences then take on a cyclic formation. This cyclic formation is crucial for the protein to remain in homeostasis and is the main source of energy transformation with bonds being broken and recreated during cyclization.
Our protein is also very
basically splits the sequence in half of prime and nonprime on either side of the split. Then, the split sequences then take on a cyclic formation. This cyclic formation is crucial for the protein to remain in homeostasis and is the main source of energy transformation with bonds being broken and recreated during cyclization.
Our protein is also very 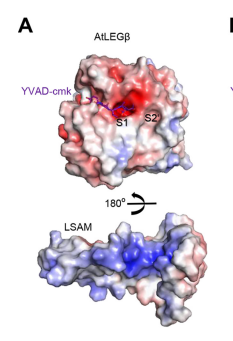
Structural highlights
Looking at the , there are 10 alpha-helices, a parallel beta-sheet, 2 anti-parallel beta-sheets, and about 7 random coils.
The parallel beta-sheet is where the interaction with the ligand (citric acid) happens. The His138 amino acid residue forms an interaction at this parallel beta-sheet.
For the , there is 1 polymer with plenty of hydrogen bonds, disulfide bridges, and hydrophobic interactions. It also contains sulfate ions, a citric acid, and a carbohydrate (glucopyranose).
The you have already seen, our protein consists of 12 total chains.
Other important features
An important feature is that proAtLEGb forms atypical dimers in the crustal and is monomeric in solutions. When compared to another form of our protein AtLEGgy findings suggest that the observed beta-dimer is weak and probably only transient in solution. 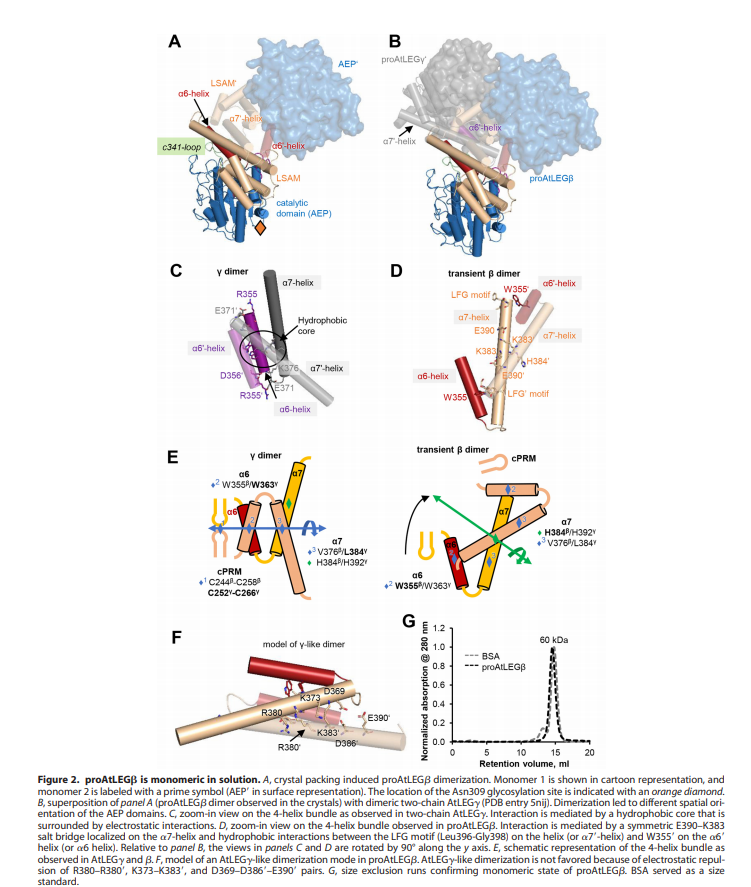
Another important feature is the cyclization of the peptides after cleavage. The cyclization is important for the cell stays programmed and is the same every time.