Introduction
TRPV1 (Vanilloid Transient Receptor Potential Type 1) is a non-selective ion channel which, in response to a stimulus, induces an incoming current of cations, primarily calcium and sodium, that causes depolarization of the cell. It is part of the TRP (Transient Receptor Potential) superfamily and is the first in a subfamily of vanilloid-sensitive TRP channels: TRPVs.TRPV1 is expressed in Group A nerve fiber and Group C nerve fiber by sensory neurons of the dorsal and trigeminal spinal ganglia. (Gastrointestinal tract’s neurons express this receptor widely which may be involved in Crohn's disease. TRPV1 is also located on the heart peri vascularisation. It has a vasodilatation effect and protects this organ via the substance it releases.) TRPV1 is implicated in nociception, its activation by heat or by chemical substances leads to a painful sensation.[1] Functional and structural studies of TRPV1 have contributed to a clearer understanding of transmission of nociceptive stimuli mechanisms especially with low diameter fibers. TRPV1 has a major role in inflammatory and pain sensitivity and overactivation of TRPV1 increases the sensitivity to pain as well as extended pain induces overexpression of TRPV1 in nerve fibers. However, sub-expression of this receptor induces chronic pain. Which can appear with abnormal neuromodulators transport due to axonal damage.
TRPV1 can be indirectly activated by NGF (nerve growth factor) and specific molecules of the inflammatory system such as bradykinin, serotonin, histamin, prostaglandin or ATP. They increase the channel opening, by blocking the PIP2 inhibition or by decreasing the heat activation threshold.
[2]
Structure of TRPV1
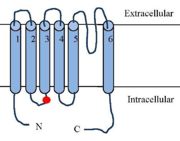
Schematic figure of the TRPV1 receptor
The TRPV1 receptor is a transmembrane protein receptor. It is made up of 839 amino acids. It’s molecular weight is 94 938Da.[3] TRPV1 exists in two states : the open state and the closed state.[4]
TRPV1 are tetrameric channel type receptors. The four subunits form a symmetry plane around a pore allowing the passage of ions.
Each TRPV1 subunits is made of one N-terminal tail, one transmembrane region, a C-terminal tail preceded by a TRP domain. The N-terminal and C-terminal region are intracellular. N and C terminal region are responsible of 70% of the total mass of TRPV1.[3]
The region has 6 repeats of .[3][5]
The transmembrane region is composed of six transmembrane a helices (,,,,,). S1,S2 and S3 helices contain aromatic side chain ().[3] A small hydrophobic domain beetween S5 and S6 with a constitutes the pore allowing the passage of ions through the TRPV1 receptor.[1][3]
Threonin residue () and Tyrosin residue () located on the fifth and the third transmembrane helices are very conserved. Threonin 550 and Tyrosin 511 are implicated in TRPV1 activation by vanilloids and in pain sensation.[6]
The S6 domain links the receptor to the domain of TRPV1. The C-terminal is made of 150 amino acids and it contains .[5].The TRP domain is made of 23-25 aminoacids with a alpha helical structure, it is found in many TRP family members.[3] TRP domain is necessary for the formation of tetrameric TRPV1.
Many amino-acids of the C-terminal domain are the target of post-translationnal modifications by kinases and phosphatases.[7]
Relation structure-function
TRPV1 is a homotetramer in which each subunit has several phosphorylation sites for PKA (protein kinase A), PKC (protein kinase C) and CaMkII (Ca2 + / calmodulin-dependent kinase II), as well as numerous glycosylation sites. These domains play a crucial role in the regulation of TRPV1 activity.
The state of the channel is modulated by two types of molecules: agents that promote its opening of the channel, called agonists, and agents that induce its closure or prevent its opening, called antagonists.
Agonists
Capsaicin
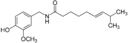
Chemical structure of capsaicin
Capsaicin is an active compound in chili.
TRPV1 receptor has a capsaicin-binding pocket formed by S3,S4 and . The capsaicin-binding pocket is surrounded by the residues .[8]
Bound capsaicin is oriented in a « tail-up, head down » configuration. In this configuration,capsaicin is anchored into the receptor.[9]
The capsaicin cycle binds via hydrogen bounds to amino acids on the S3 helix (), on the and on the S6 helix (). The amid group of capsaicin binds the helix.[5].
Capsaicin maintains TRPV1 in an open state. A conformational change wave spread over the whole pore.[10]. This leads to the massive enter of Ca2+ and Na+ and the depolarization of the nerve fiber. Depolarization triggers the generation of an action potential causing a painful sensation.[1] Ca2+ has a negative retrospection effect on TRPV1 and induces a desensitization of this receptor facing capsaicin.
[2]
Resiniferatoxin (RTX)
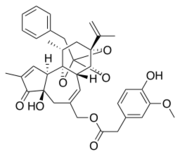
Chemical structure of resiniferatoxine
Resiniferatoxin is a natural analogue of capsaicin. It is the most potent TRPV1 agonist known, with a binding affinity for TRPV1 ~500x higher than that of capsaicin.
The pocket size characteristics of the TRPV1-RTX allow the installation of RTX : The sub-pocket near is shallow in the TRPV1-RTX because and are close and is oriented towards the vanilloid pocket.[11]
The sub-pocket near , and accommodates the diterpene group of the RTX.[11]
The aromatic part of resiniferatoxin is located deeper in the sub-pocket near and is oriented almost parallel to the aromatic side chain of , so it establishes a strong interaction π-π. The aromatic hydroxyl and methoxy groups of the RTX form strong hydrogen bonds with , and . The ester group is linked to and by hydrogen bonds.
Regulation
Sensitization
Phosphorylation of the TRPV1 receptor leads to its sensitization
(the process regulates TRPV1 its functionality). Phosphorylations are either caused by PKC (IP3 signalling), by PKA (AMPc signalling), or by CamKII activation by inflammatory mediators. [5] Depending on the target residu, the impact on the receptor will be different as various activation pathways are impacted. Phosphorylation improves the efficiency of the receptor and because it regulates the function, it is a target to treat Hyperalgesia. [12]. PKA phosphorylates , PKC and CaMKII phosphorylate .
The phosphorylation of TRPV1 lead to an over-expression of TRPV1 at the membrane surface.[13] Moreover, phosphorylated TRPV1 would have a reduced channel opening threshold.[14]. As a result phosphorylated TRPV1 are more responsive to agonist and the resulting pain sensation is higher.
PKA phosphorylates , PKC and CaMKII phosphorylate .
Desensitization
A repeated exposure of TRPV1 to capsaicin fails to activate the receptor. It occurs by a Ca2+-dependent mechanism that leads to a desphosphorylation by the calcineurin phosphatase of the serine and threonine residues which have been previously phosphorylated by PKA (). Thus, the decrease in TRPV1 phosphorylation diminish the sensitivity of the capsaicin channel and leads to a decrease in capsaicin's response by negative feedback.
The over-stimulation of TRPV1 is followed by the nerve endings' death due to calcium overload, causing analgesia. [5]
Implication of TRPV1 in the treatment of pain
Capsaicin stimulates and desensitizes several receptors from the A(delta) and C fibers. This phenomenon will release inflammatory neuropeptides.
Capsaicin assists the entry of Ca2+ in the neuron by a nonspecific membrane.
TRPV1 can be activated by heat and voltage variation.
This receptor exists under 3 different forms and ethanol is able to activate this receptor.
In 2011 Qutenza (NeurogesX) patch containing 8% of capsaicin has been marketed in France and indicated in the neuropathic pain.
The absorption through the skin of these creams generated partial desensitization of the nerve endings. This is the cause of a decrease in painful sensations.[15]
Capsazepine is a synthetic competitive antagonist of this receptor which is currently used to study TRPV1 function.
Many laboratories are conducting clinical studies on oral TRPV1 antagonists: GlaxoSmithKline , Amgen, Merck-Neurogen, Abbott, Eli Lilly, AstraZeneca and Japan Tobacco. Nowadays at least seven orally active TRPV1 antagonist substances successfully went for clinical development and the laboratories cited before all completed phase I trials . However some of them have stopped their researches at phase II trials by unknown reason as GlaxoSmithKline
with the antagonist SB-705498 or Lilly with the antagonist GRC 6211. [16] [17]
The research on the antagonists of TRPV1 remains encouraging. [18]


