Sandbox Reserved 1658
From Proteopedia
| This Sandbox is Reserved from 26/11/2020, through 26/11/2021 for use in the course "Structural Biology" taught by Bruno Kieffer at the University of Strasbourg, ESBS. This reservation includes Sandbox Reserved 1643 through Sandbox Reserved 1664. |
To get started:
More help: Help:Editing |
|
Contents |
Generalities
Protein : Neuropilin-1
Gene : NRP1
Organism : Homo sapiens (Human)
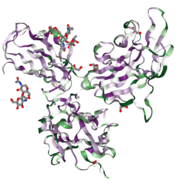
Neuropilin is a highly conserved type I [1] transmembrane protein (see image on the right, determined by ConSurfDB).
Two different types of Neuropilin have been discovered in vertebrates: Neuropilin-1 (NRP1) and Neuropilin-2 (NRP2). They have a similarity of 44 % between their amino acid sequences[2]. In the human genome, Neuropilin-1 is located on chromosome 10, with the molar weight fluctuating between 120 and 130 kDa[2].
Neuropilins are prominently found in the membrane of endothelial cells, but Neuropilin-1 is additionally involved in several processes such as axon guidance during the embryonic development, recognition of the Vascular Endothelial Cell Growth Factor (VEGF) and recognition of COVID-19[3].Structure
Neuropilin-1 has three different domains[2]: a cytoplasmic domain containing 40 residues, a transmembrane domain containing 24 residues, and a 850 residue ectodomain[4]. The latter is an assembly of five individual motifs (a1, , , and c). These can be further subdivided into the CUB domains (a1 and a2), two homologous domains to coagulation factors V/VIII (b1 and b2) and a MAM domain (c). Ligand binding is mediated by the CUB domains and the (b1/b2) portion of the ectodomain while the c domain mediates Neuropilin oligomerization. However, the c domain is not able to independently facilitate multimerization of NRP molecules. It has therefore been hypothesized to contribute to the assembly and regulation of the signaling complexes by positionning the other extracellular domains of Neuropilin-1 away from the membrane.[5]
As an example, the semaphorins (SEMA) bind to the CUB domains and b1 while vascular endothelial growth factors (VEGFs) bind to b1 and b2 [2]. The c domain, as well as the transmembrane domain, is involved in receptor dimerization. The cytoplasmic domain contains a PDZ domain rather than a binding site. This segment is only 42-44 amino acids length and has no catalytic function. It participates in the formation and stimulation of signalling complexes.
It has been determined that the interactions between b1 and b2, as well as between a2 and b1/b2 are the same for Neuropilin 1 and 2. However, the interactions between a1 and the other domains are not yet fully understood. The a1 and a2 domains contain [2]. The ion is coordinated by two carbonyl oxygens from Ala(252)and Ile(253) and by three negatively charged side chains: (Glu(195), Asp(209) and Asp(250))[2]. On the other side, b1 and b2 form a jellyroll[2] composes of 8 beta-sheets ().One part of the domain contains three loops that typically constitute the ligand binding site for members of the Discoidin domain family.
Function
Neuropilins are involved in several signaling pathways. They act mainly as co-receptors because of their small cytoplasmic domain, and therefore associate with other receptors to transduce their signals through a cell membrane. Neuropilin-1 is involved in the development of the cardiovascular system, but also in angiogenesis and organogenesis. It is also involved in the development of some neuronal circuits.
In cardiovascular development
In mature organisms, neuropilins primarily perform the role of pro-angiogenic co-receptors[6]. Neuropilin-1 works as a specific co-receptor of VEGFR-2 for VEGF-A. After binding and activation, neuropilins promote angiogenesis by stabilizing the VEGF/VEGFR[6] binding. Researchers also think that neuropilins affect the vascular motility of endothelial cells, independently of their action on the protein complex VEGF/VEGFR. Besides, NRP1 enhances the signalling of the extra-cellular matrix in endothelial cells.
In neuronal development
In neuronal tissues, NRP1 associates with semaphorin-3A to perform growth cone guidance. It helps guiding axonal growth during the development of the nervous system by mediating the chemorepulsant activity of semaphorin.
In the immune system
Expression of NRP1 has been detected in several cells of the immune system[7] such as macrophages, dendritic cells, as well as in T cell subsets. In dendritic cells and T cells subsets, NRP1 helps to trigger the immune response. It has also been hypothesized that NRP1 could represent a new activation marker for T cells. NRP1 is particularly involved in the interaction between T cells and dendritic cells. It is also involved in the removal mechanism of T cells and in the transmission mechanism of immunoregulatory effects of semasphorin-3A on T cells.
Disease
Since neuropilins are involved in the development of the neuronal and cardiovascular system, abnormalities in these genes lead to abnormalities in cardiac, vascular and nervous development. A dysregulation of the activity of neuropilins is involved in many pathologies, such as cancers and cardiovascular diseases. Indeed, neuropilins stimulate many functions that increase tumor aggression such as immune tolerance or cell proliferation. For example, overexpression of NRP1 has been detected in many cancers, including leukemia, lymphoma and melanoma[8]. It would stimulate migration, invasion and tumorigenesis. The role of NRP1 as a mediator of tumor development has been investigated and many observations show that overexpression of NRP1 is also involved in colon[9] cancer, breast cancer, lung cancer and glioma.
Role in covid contamination
Neuropilin-1 is one of the entry sites of the SARS-CoV-2 in cells. Autopsies have shown SARS-CoV-2 to infect NRP1-positive cells[3] facing the nasal cavity. Unlike SARS-Cov, SARS-CoV-2 contains a polybasic furin-type cleavage site[3] at the S1-S2 junction in the spike protein(S). Previously it had already been known that NRP1 binds furin-cleaved substrates. The cleavage of the spike protein causes the formation of a C-terminal motif which observes the Cend rule. This motif is responsible for the binding of the virus on the b1 domain of NRP1. Therefore, Neuropilin-1 facilitates the entry of Sars-Cov-2 in cells.
Applications
NRP1 targeting is investigated as a possible cancer therapy [10]. Several methods have been developed to inhibit the oncogenic activities of NRP1 by using iRNA [11], monoclonal antibodies or even peptides. Especially, monoclonal antibodies are experimented as antitumor agents. Some have already being developed such as specific CUB antibodies[11] and anti-NRP1B[11] that inhibit cell migration induced by VEGF and the formation of tumors in endothelial cells.
References
- ↑ Fumio Nakamura and Yoshio Goshima Bookshelf ID: NBK6408 https://www.ncbi.nlm.nih.gov/books/NBK6408/
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 Appleton BA, Wu P, Maloney J, Yin J, Liang WC, Stawicki S, Mortara K, Bowman KK, Elliott JM, Desmarais W, Bazan JF, Bagri A, Tessier-Lavigne M, Koch AW, Wu Y, Watts RJ, Wiesmann C. Structural studies of neuropilin/antibody complexes provide insights into semaphorin and VEGF binding. EMBO J. 2007 Nov 28;26(23):4902-12. Epub 2007 Nov 8. PMID:17989695
- ↑ 3.0 3.1 3.2 Cantuti-Castelvetri L, Ojha R, Pedro LD, Djannatian M, Franz J, Kuivanen S, van der Meer F, Kallio K, Kaya T, Anastasina M, Smura T, Levanov L, Szirovicza L, Tobi A, Kallio-Kokko H, Osterlund P, Joensuu M, Meunier FA, Butcher SJ, Winkler MS, Mollenhauer B, Helenius A, Gokce O, Teesalu T, Hepojoki J, Vapalahti O, Stadelmann C, Balistreri G, Simons M. Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science. 2020 Nov 13;370(6518):856-860. doi: 10.1126/science.abd2985. Epub 2020, Oct 20. PMID:33082293 doi:http://dx.doi.org/10.1126/science.abd2985
- ↑ Christian C. Lee, Andreas Kreusch,Daniel McMullan, Ken Ng, and Glen Spraggon Crystal Structure of the HumanNeuropilin-1 b1 Domain https://www.cell.com/structure/pdf/S0969-2126(02)00941-3.pdf
- ↑ Yelland T, Djordjevic S. Crystal Structure of the Neuropilin-1 MAM Domain: Completing the Neuropilin-1 Ectodomain Picture. Structure. 2016 Oct 3. pii: S0969-2126(16)30267-2. doi:, 10.1016/j.str.2016.08.017. PMID:27720589 doi:http://dx.doi.org/10.1016/j.str.2016.08.017
- ↑ 6.0 6.1 Guo HF, Vander Kooi CW. Neuropilin Functions as an Essential Cell Surface Receptor. J Biol Chem. 2015 Dec 4;290(49):29120-6. doi: 10.1074/jbc.R115.687327. Epub 2015 , Oct 8. PMID:26451046 doi:http://dx.doi.org/10.1074/jbc.R115.687327
- ↑ Roy S, Bag AK, Singh RK, Talmadge JE, Batra SK, Datta K. Multifaceted Role of Neuropilins in the Immune System: Potential Targets for Immunotherapy. Front Immunol. 2017 Oct 10;8:1228. doi: 10.3389/fimmu.2017.01228. eCollection, 2017. PMID:29067024 doi:http://dx.doi.org/10.3389/fimmu.2017.01228
- ↑ Jubb AM, Strickland LA, Liu SD, Mak J, Schmidt M, Koeppen H. Neuropilin-1 expression in cancer and development. J Pathol. 2012 Jan;226(1):50-60. doi: 10.1002/path.2989. Epub 2011 Oct 25. PMID:22025255 doi:http://dx.doi.org/10.1002/path.2989
- ↑ Parikh AA, Fan F, Liu WB, Ahmad SA, Stoeltzing O, Reinmuth N, Bielenberg D, Bucana CD, Klagsbrun M, Ellis LM. Neuropilin-1 in human colon cancer: expression, regulation, and role in induction of angiogenesis. Am J Pathol. 2004 Jun;164(6):2139-51. doi: 10.1016/S0002-9440(10)63772-8. PMID:15161648 doi:http://dx.doi.org/10.1016/S0002-9440(10)63772-8
- ↑ Chaudhary B, Khaled YS, Ammori BJ, Elkord E. Neuropilin 1: function and therapeutic potential in cancer. Cancer Immunol Immunother. 2014 Feb;63(2):81-99. doi: 10.1007/s00262-013-1500-0. , Epub 2013 Nov 22. PMID:24263240 doi:http://dx.doi.org/10.1007/s00262-013-1500-0
- ↑ 11.0 11.1 11.2 Ding Y, Zhou J, Wang S, Li Y, Mi Y, Gao S, Xu Y, Chen Y, Yan J. Anti-neuropilin-1 monoclonal antibody suppresses the migration and invasion of human gastric cancer cells via Akt dephosphorylation. Exp Ther Med. 2018 Aug;16(2):537-546. doi: 10.3892/etm.2018.6234. Epub 2018 May, 30. PMID:30116312 doi:http://dx.doi.org/10.3892/etm.2018.6234