LntA protein
Listeria monocytogenes is an ubiquitous Gram + bacteria and is the only kind of Listeria which is pathogenic for humans. In fact, it is responsible for the human listeriosis which can range from gastroenteritis to fatal meningitis It It is a rare but severe illness for pregnant women, elderly and immuno-compromised subjects with a death rate around 30%. During infection, the bacterium penetrates the cell and secretes multiple virulence factors that modulate the host's gene expression. LntA is one of these virulence factors and it targets Interferons-stimulated genes.[1]
While interferons (IFNs) are able to limit viral infections, their role in bacterial infection remains unclear. In the case of Listeria, the expression of interferons-stimulated-genes (ISG) enhances its pathogenicity. Lebreton and a.l solved the 3D structure of LntA by x-rays crystallography (PDBID: 2XL4) in order to elucidate the molecular mechanisms of the host's transcriptional machinery[2].
Function
Once the bacteria reaches the host's cytoplasm, the expression of LntA is activated, the protein is excreted and addressed to the nucleus thanks to a peptide signal. Then, LntA interacts with the transcription factor BAHD1. In absence of infection, BAHD1 represses the expression of ISG by promoting the local formation of heterochromatin while the interaction of LntA with BAHD1 has the effect of removing the chromatin repressor from the host’s DNA. Therefore, L.monocytogenes virulence factor induces a strong interferon response which enhances its pathogenicity[3].
The mechanisms by which Listeria benefits from the synthesis of interferons are not fully understood. One hypothesis could be that Listeria monocytogenes takes advantage of the arrest of cellular-cycle induced by interferons. [1] Indeed, this mechanism could be similar to those used by other pathogens such as Salmonella [2] or Yersinia [3] which are able to promote an inflammatory response in gut epithelium in order to facilitate their dissemination and colonization.
In addition, Lebreton et al showed that when listeria grows outside the cell, the transcription rate of LntA is almost null and that a constitutive expression of LntA has an antibacterial effect. Thus, the efficiency of LntA requires a precise temporal and quantitative regulation.
Structure
LntA is a small basic protein of 9.7 kDa. This protein is highly conserved in L. monocytogenes and is absent in almost all non-pathogenic Listeria strains . This characteristic suggests that lntA plays a key role in Listeria’s virulence. The acidic part of LntA is composed of aspartic acid () and the basic part is composed of lysine and arginine (). LntA folds in a compact helical structure and is composed of 5 alpha-helix, three of them are long antiparallel helix and can be seen as the core of the protein. The two remaining helix stick out the core. The 3 first helix are named , and . The 2 others are and . These residues located in display high RMSD values meaning that this region is likely to oscillate. In fact, by studying this protein on pymol we can see it by the thickness and redness of these helixes which means that they have a high RMSD value :
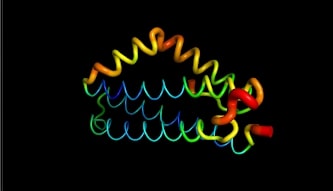
The flexibility of and may have a role in the binding to BADH1. Indeed, the positioning of this "elbow" relatively to the "trunk" formed by the less mobile regions , and is determining in the ligands recognition. Many amino acids may be involved in the interaction of LntA with its ligand, such as BAHD1. A has proven to be essential for the interaction with the transcription factor BAHD1 thanks to a conformational change . Indeed, when this motif is substituted by two aspartic acid amino acids (K180D/K181D by mutation of LntA), a local redistribution of the charges is observed and lntA is not able anymore to interact with BAHD1. [4]
Electrostatic views have shown that the surface of LntA presents three distinct major charged patches that are involved in the the interaction with protein partners such as (BAHD1) : Patch 1 is highly acidic, Patch 2 is a basic region and Patch 3 is a second highly basic region especially containing the .
This protein can also be stabilized by glycerol molecules because they are hydrophobic and it prevents hydrolyzation.
Furthermore, the structure of LntA with mutated K180 - K181 (into D180 - D181) was also solved [4] and more recently, the structure of the whole protein was predicted by alpha fold 2.
Nevertheless, the structure of the complex LntA-BAHD1 is not entirely resolved yet. [5]
Conclusion
The discovery of the LntA virulence factor shows that pathogenic bacteria can implement complex infectious strategies, requiring very precise temporal and quantitative regulation of the virulence factor delivery. Studies on virulence factors that target the nucleus can lead to the discovery of new mechanisms of gene expression regulation. And more importantly, the understanding these dialogs might allow new drug design and possibly a better support of the patients. Furthermore, the awareness of such mechanisms is very recent and raises many questions. For instance, it is still unclear whether the impact of lntA on gene expression is merely temporary or whether it leaves epigenetic marks over the long term.
Key points about LntA: Function:
- Is a virulence factor of L. monocytogenes
- Interacts with BAHD1, a transcription factor, in the nucleus
- Remove the chromatin repressors from the host's DNA
- Triggers the formation of interferons
Structure:
-A 9.7 kDa protein
-Made up of five alpha-helixes
-H4 and H5 responsible of the interaction with BAHD1

