MRGPRX2 is a certain type of GPCR that is located in the cellular membranes of mast cells.
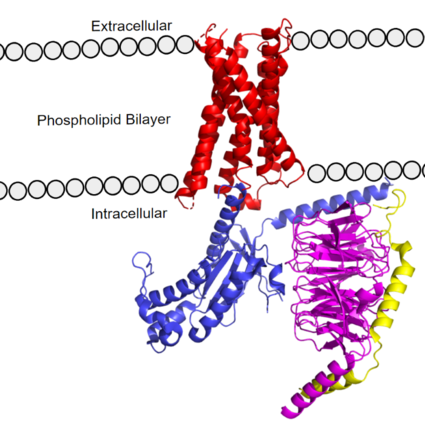
Figure 1. MRGPRX2 as it sits within the cellular membrane. Phospholipid bilayer is represented by grey dots, with labeled cellular locations. PDB: 7s8l
Background
GCPR’s or G-Protein Coupled Receptors are a large family of protein receptors that promote cellular signaling and signal transduction[1]. GPCRs transmit extracellular signals to intracellular messages. Many essential pathways utilize GPCRs, including human vision by the GPCR Rhodopsin, and the adrenaline fight-or-flight response by the β2-adrenoceptor GPCR. Understanding GPCR’s and how they produce their desired intracellular signal is essential to studying essential cellular pathways, especially in their diseased states. GCPRs are common drug targets, with 475 drugs acting on over 100 GPCRs. An additional 300 drugs are in clinical trial stages, and 20% of those drugs are targeting novel GPCRs [2]. Because of the clinical relevance of GPCRs, new structures provide new avenues for drug development to both treat disease or modulate the harmful side effects.
Some cells in the human body that express the MRGPRX2 receptor include mast cells in the skin, intestines, and trachea [3][4]. Mast cells are immune cells responsible for triggering inflammatory responses. Mast cells are densely packed with granules containing inflammatory chemicals, such as histamine and heparin[4]. Mast cells can be activated by either antibodies from the immune response or upon ligands binding to MRGPRX2 receptors on their surface[5]. Upon activation, mast cells will release histamine-containing granules which can trigger a larger inflammatory response [4][5]. These responses induce common allergic reaction or anaphylaxis symptoms, such as cutaneous itching sensations or airway constriction[6][7][5].
Ligands that bind to MRGPRX2 in the natural environment to produce an allergic response include exogenous molecules, some being contents of insect venom, molecules like Compound 48/80 (C48/80), or other polycationic molecules[4][3]. They can also respond to endogenous signaling molecules involved in inflammation pathways such as cytokines, anaphylatoxins, or neuropeptides[3]. Many pseudo-allergic drug reactions have been tied to overactivity of MRGPRX2 receptors on mast cells[5], so research into receptor-ligand interactions of the MRGPRX2 receptor has the potential to mediate many adverse itching and allergic reaction side effects seen in drugs today[5].
GPCRs are categorized into 6 different classes based on shared sequences and functions. MRGPRX2 is categorized into the Class A receptor family. However, itch receptors like MGPRX2 have unique structural features from most class A receptors [6][7]. These unique structural features, as seen in Class A Family Differences, cause conformational changes throughout the protein that impact what ligands bind to the receptor[7].
GPCR Structure
The MRGPRX2 receptor structure was determined by cryo-electron microscopy (cryo-EM) [6] [7]. Despite MRGPRX2’s novel characteristics, these structures still confirmed MRGPRX2 classification as an A-family GPCR. MRGPRX2 therefore shares the same general structural domains of all GPCR’s. This includes a that interacts with a heterotrimeric domain, consisting of , , and subunits. The G-protein serves as the intracellular relay for ligand binding to the receptor. In preparing the protein sample, MRGPRX2 was prepared with an in order to stabilize the transmembrane domain for proper imaging. For simplicity and to focus on the MRGPRX2 receptor, the antibody has been removed in structural scenes.
Transmembrane Domain
The transmembrane domain spans the cell membrane (Figure 1) and it consists of and (three extracellular loops, and three intracellular loops). The transmembrane helices are numbered 1-7 and contain special conserved motifs that are shared across other A family receptors. These motifs are expanded upon later, as they heavily contribute to the structure and therefore function of the transmembrane domain as a whole.
The extracellular region of the 7 transmembrane domain forms a single binding pocket with . Sub-pocket 1 is negatively charged due to negatively charged residues (Asp-184 and Glu-164), while sub-pocket 2 contains hydrophobic amino acids which contribute to hydrophobic interactions between the ligand and protein. The intracellular region (Figure 1) is what connects the transmembrane helices with the G-protein.
This GPCR has been modeled both as MRGPRX2 and MRGPRX4[6][7], though much of this page focusses on MRGPRX2. X4 is found to mediate cholestatic itch compared to X2's regulation of mast cell degranulation and hypersensitivity itch-reactions[6]. X4 and X2 demonstrate nearly the same structural differences compared to that of other class A GPCRs. Interestingly, X4 can interact with negatively charged bile acids and is insensitive to the common X2 cationic agonists discussed later (Figure 7).
G-Protein
GTP-binding proteins, also known as G-proteins, are heterotrimeric complexes consisting of , , and subunits that interact with the intracellular transmembrane region at an ( Figure 2b). G-proteins are responsible for transmitting extracellular signals into the cell upon activation. Activation leads to a substitution of GDP with GTP within the alpha subunit, causing the alpha subunit to disassociate from the beta and gamma subunits to initiate an intracellular signaling cascade. There are different families of G-alpha subunits, Gαi, Gαs, Gα12/13, and Gαq [8]. MRGPRX2 binds to both Gαi and Gαq subunits with nearly identical structures despite slightly different amino acids present ( Figure 2a) [6] [7]. Throughout this page, MGPRX2 is always shown with Gq. The major difference between the Gq and Gi bound structures comes from one amino acid difference (valine on Gq versus phenylalanine on Gi) that pushes the Gi subunit 2Å away from the arginine residue on helix 6 of the transmembrane protein.
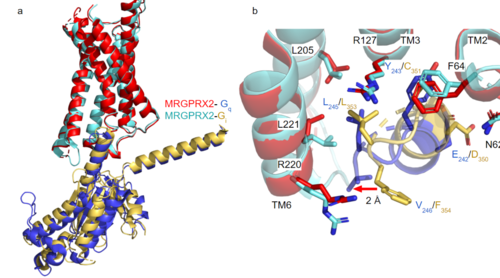
Figure 2a. Overlay of MGPRX2-Gq (red-dark blue) and MGPRX2-Gi (cyan-yellow).
Figure 2b. Important residues involved in the interface between MGPRX2 and Gq/ Gi subunits. Arrow pointing to the major difference between the interfaces, which comes from the final C-terminus residue on the G-alpha subunit. In Gq, there is a valine while in Gi, there is a phenylalanine. This pushes the Gi subunit 2Å away from the arginine residue on helix 6 of the transmembrane protein. All other interactions are nearly identical. PDBs: (MRGPRX2-Gq):7s8l and (MRGPRX2-Gi):7s8m
Class A Family Differences
The MRGPRX2 receptor shows surprising differences between it and all other previously characterized class A GPCRs including many conserved class A structural motifs which are absent on MRGPRX2. These structural motif differences contribute to a ligand binding site closer to the membrane surface for MRGPRX2 rather than a ligand binding site deep within the helices (Figure 3). To demonstrate this difference and other structural differences, an of MRGPRX2 and 5-HT2AR, another class A GPCR with more conserved structural motifs is provided.
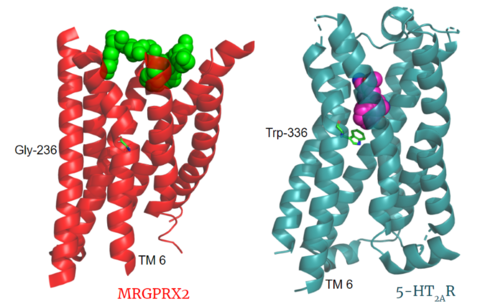
Figure 3. Comparison of ligand Cortistatin-14 binding in MRGPRX2 (left) and binding of 25-CN-NBOH (hallucinogen) in 5HT2AR (right). PDBs: (MRGPRX2): 7s8l and (5HT2AR): 6wha
Toggle Switch
Key toggle switch residues in the ligand binding pocket can act as molecular switches to turn the GPCR “on” or “off". Toggle switches initiate the transmission of the molecular signal through the 7 TMD helices to the intracellular G protein. Trp-336 is the "iconic" toggle switch in class A GPCR’s [9], and is a part of another motif, known as the CWxP motif. However in MRGPRX2, this tryptophan has been replaced with a [6] [7]. This shift leads to a significant modification to the receptor structure. By replacing the large tryptophan residue with a small glycine, the membrane helices, especially helix 7 on which the toggle switch is found, pack more tightly. The ligands that interact with MRGPRX2 are able to bind much of the receptor, as opposed to deeper within the helices (Figure 3). This shallower binding pocket expands the types of ligands that are able to interact with X2 and therefore what types of molecules can activate the Human Itch GPCR. More details about what kinds of ligands bind to this receptor are discussed later.
Sodium Site
The allosteric sodium site in class A GPCRs has been characterized as important in inactive state GPCR stabilization [10]. Katritch et al [10] describe that class A GPCRs lacking conserved D2.50 and other polar residues within the sodium pocket are typically inactive. The MRGPRX2 consists of conserved D2.50, or ASP-75, and GLY-116 compared to the previously conserved polar residues in this binding pocket such as S3.39. Other class A GPCRs demonstrate a larger sodium binding pocket with a higher negative character allowing for a suitable environment for sodium ions to bind. In MRGPRX2, this sodium binding pocket lacks the same amount of with the shift to a glycine residue rather than serine. However, evidence suggests that sodium is still able to bind in X2's sodium binding site even with fewer conserved residues.
PIF/LLF Motif
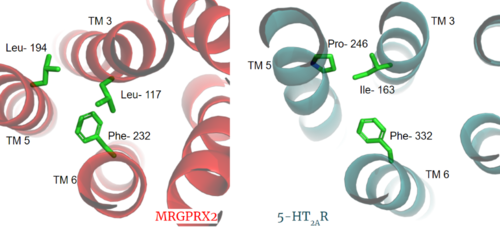
Figure 3. Conserved PIF motif in 5HT2AR (teal) compared to the LLF motif found in MRGPRX2 (red). Transmembrane helices and residues are numbered and labeled to show how this structural change shifts the orientation of the helices. PDBs: (MRGPRX2): 7s8l and (5HT2A): 6wha.
Another motif found in most, but not all, A family GPCR’s is the PIF motif. The PIF residues () are found on transmembrane helices 5, 3, and 6, respectively. In MRGPRX2, the PIF motif is changed to LLF residues. Figure 3 shows the conserved PIF motif on 5HT2AR, to the LLF motif on MRGPRX2. This change to LLF shifts helix 6 towards helix 3, and contributes to the tighter packing of helices [6] [7] and therefore a more surface-level ligand binding site.
DRY/ ERC Motif

Figure 4. ERC Motif of MRGPRX2 with key residues shown as ball and stick. PDB: 7s8l.
The E/DRY motif in most class A GPCRs is responsible for forming salt bridges with surrounding residues and TM6[11]. These salt bridges maintain the inactive conformation of the receptor until ligand binding breaks the ionic "lock" from these interactions. MRGPRX2 has an ERC motif rather than the typically conserved E/DRY Motif. The amino acid residue shift from TYR-174 to CYS-128 allows compaction of the helices in MRGPRX2 where the standard TYR physically pushes the TMD helices apart(Figure 4). The conserved residues E and R still form salt bridges with nearby residues. This and the closer packing of the helices contribute to a less significant TMD conformational change upon ligand binding (Figure 10).
Disulfide Bonds

Figure 5. Overlay of the 5HT2AR and MRGPRX2 TMP for comparison of disulfide bond location. PDBs: (MRGPRX2): 7s8l and (5HT2A): 6wha.
In a large majority of class A GPCRs, there is a conserved disulfide bond between extracellular loop 2 (ECL2) and transmembrane helix 3 (TM3). This bond has been proposed to have a role in structural stability, expression, and function of GPCRs[12]. The MRGPRX2 disulfide bond is between on TM helices 5 and 4, respectively. For example, the shows this disulfide bond between the ECL2 and TM3. Although this bond is in a different location than other class A GPCRs, there is evidence to suggest its location is essential for the signaling of the X2 receptor as the ECL2 instead located at the top of TM4 and TM5 allowing for the large, extracellular binding pocket observed in X2[6].
Further Information
The MRGPRX2 GPCR also contains semi-conserved or fully-conserved motifs that are seen in other class A GPCRs.
NPxxY Motif
The residues in the are pivotal for receptor activation in all Class A GPCRs. This motif is conserved in the MRGPRX2 receptor with residues VAL-231, ASP-75, ASN-275, and TYR-279.
CWxP Motif
The is almost fully conserved except for TRP-236, or the toggle switch, which is replaced with GLY-236. CYS-235, LEU-237, and PRO-238 are all conserved. CYS6.47 may play a fundamental role in GPCR activity by participating in the rearrangement of TM6 and TM7 upon receptor activation[13]. This residue's role is supported by its conservation in MRGPRX2 as it is a functional GPCR.
Ligands
MRGPRX2 binds a wide range of small molecule and peptide ligands. These ligands have positively charged regions which can interact with the negative binding pocket, sub-pocket 1. Some of these larger ligands also have a hydrophobic region which can interact with sub-pocket 2. MRGPRX2 is activated by C48/80[7][6] and LL-37[4], among other endogenous inflammatory chemicals.
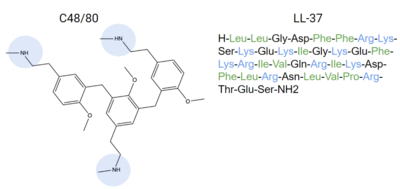
Figure 6. Endogenous MRGPRX2 ligands with positive regions in blue and hydrophobic regions in green. C48/80 and LL-37 are both released by the body as an allergic reaction response
[4]. These compounds can bind to MRGPRX2 and activate it, explaining how itching is a side effect of allergic reactions.
Other endogenous compounds that share similar characteristics of these ligands are Zinc-3573, PAMP-12, and Cortistatin-14 (Figure 7). These molecules are known agonists of MRGPRX2 and bind similarly to sub-pockets 1 and 2.
An endogenous peptide agonist, models these interactions, binding to sub-pocket 1 through LYS-3 and sub-pocket 2 through hydrophobic interactions.
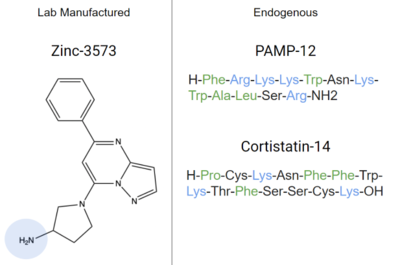
Figure 7. Agonists of MRGPRX2 due to their structural similarities to known ligands. Positive regions shown in blue and hydrophobic regions in green. Cortistatin-14, PAMP-12, and Zinc-3573 are all known to bind to MRGPRX2 with high affinity, and inhibit mast cell degranulation
[7][6]
Function

Figure 8.Schematic representation of cellular response
GPCRs undergo a conformational change in their 7TMD region upon ligand binding. This signal is then transduced to the G-protein allowing for downstream responses due to between the alpha subunit of the G-protein and the transmembrane protein which activates g-protein by GTP exchange. This downstream response may be in the form of release of small granules which can be received by a receptor to initiate a pathological response (Figure 8)[3].
Before Activation
The culmination of different motifs observed in MRGPRX2 compared to other class A GPCRs leads to external membrane ligand binding. The MRGPRX2 GPCR undergoes a much smaller conformational change upon ligand binding compared to other Class A GPCRs due to surface level binding versus deep helix binding (Figure 9). This smaller change can be seen as only minor extracellular movement occurs in MRGRPX2 upon binding compared to other GPCRs which can undergo loop and helical conformational changes[14].
After Activation
After ligand binding and transmembrane protein activation, this signal is transmitted to the alpha subunit of the G-protein which undergoes chemical and conformational changes. The alpha subunit is initially bound with GDP which is then physically replaced by GTP leading to conformational changes. These changes can be seen in this video of a G-protein alpha subunit activation derived from common rats. This video was constructed by one of the page's authors using PDB files 1bof and 1cip.

Figure 9. Overlay of unbound (transparent) and bound (opaque) transmembrane proteins of both MRGRPX2 (left) and 5-HT2AR (right). PDBs: (MRGPRX2): 7s8l and (5HT2A): 6wha.
Clinical Relevance
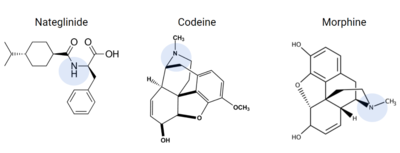
Figure 10. Common prescriptions that are tied to adverse itching side effects include nateglinide, morphine, and codeine. These drugs have structural similarities to known ligands for the MRGPRX2 receptor, and can lead to MRGPRX2 activation.
Many medications commonly list chronic skin itching or inflammatory responses as side effects, such as nateglinide, an anti-diabetic drug [6]. Other prescribed medications that list itching as a possible side effect are Atracurium, Rocuronium, Ciprofloxacin, and Levofloxacin[15], all of which are tied to MRGPRX2 activation.
Other drugs that have been known to trigger allergic reactions and even anaphylactic responses are opioids morphine or codeine[6], which could explain anaphylactic responses to anesthetics seen in some patients[5]. Upon analysis of these drugs, they share many structural similarities that are known to bind to the sub-pockets in MRGPRX2, shown in Figure 10, which is a possible explanation for the unwanted itch and inflammation responses produced in some patients when administering these drugs [5][6].
Possible treatments for these unwanted side effects of drugs can be developed by understanding the mechanism of the MRGPRX2 receptor. Research on Cortistatin-14, an endogenous neuropeptide (Figure 7), has shown that it has anti-inflammatory properties because of how it binds to the MRGPRX2 receptor [16]. Additionally, Osthole, an extract from Cnidium monnieri plants, also known as Monnier's snow parsley, demonstrates similar MRGPRX2 inhibition because of its structural similarity to many known ligands for this receptor, and could possibly be used as a treatment option for adverse allergic reactions to commonly prescribed drugs[4]. More research on MRGPRX2 can open the door for the development of drugs that avoid activation of the MRGPRX2 receptor, or possible treatments for excessive inflammation and allergic reactions.
3D Structures
7s8l, MRGPRX2 Gq
7s8m, MRGPRX2 Gi
6wha, 5HT2AR











