Sandbox Reserved 1741
From Proteopedia
| This Sandbox is Reserved from August 30, 2022 through May 31, 2023 for use in the course Biochemistry I taught by Kimberly Lane at the Radford University, Radford, VA, USA. This reservation includes Sandbox Reserved 1730 through Sandbox Reserved 1749. |
To get started:
More help: Help:Editing |
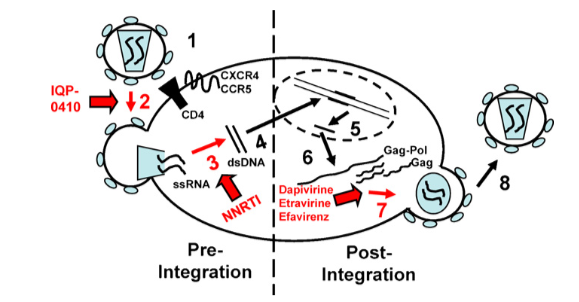
HIV-1 Reverse TranscriptaseReverse Transcriptase (RT), also known as RNA-Directed DNA Polymerase, is an enzyme used by retroviruses for the catalysis of the transcription of retrovirus RNA into DNA, which is the reverse of normal cellular transcription (DNA to RNA). HIV-1 RT is the reverse transcriptase used by the Human Immunodeficiency Virus and is what makes the virus so infectious once in the body. The discovery of HIV-1 RT was a landmark discovery, and the first drugs offered to people living with HIV-1 were inhibitors of HIV-1 RT (1). FunctionHIV is a retrovirus, which means that it only carries single stranded RNA, and so relies on reverse transcription to propagate itself to new cells (2). HIV reverse transcriptase (RT) is a heterodimer consisting of a p51 and a p66 subunit, the latter of which contains catalytically active DNA polymerase and RNase H domains, essentially functioning as a catalyst for the reverse transcription reaction (3). The enzyme RT is responsible for turning the infectious single-stranded ssRNA genome into double-stranded dsDNA provirus to integrate the virus into a new host cell (4). HIV integrase then integrates it into the host chromosome. The retro transcription process begins when the HIV particle fuses with the membrane of the host due to interactions between the envelope of the glycoprotein and coreceptors on the surface of the host cell (5). The infectious content of the HIV particle is then released into the cytoplasm of the host cell. Here the ssRNA serves as a template for HIV RT to form the dsDNA provirus. The dsDNA is then imported into the nucleus, which is then integrated into the host chromosome by another enzyme, HIV integrase. Once the dsDNA is integrated into the nucleus it makes modified mRNA that code for viral proteins and new ssRNA gnomes that combine to form new viral HIV particles (4). Some viral proteins that are made by the modified mRNA include Gag, Gag-Pol, and Env. The modified mRNA also codes for the new accessory proteins Nef, Vif, Vpr, and Vpu. (6) These viral proteins then form chains, which combine with the ssRNA to form new viral particles. These particles then release (bud) from the cell, taking some of the cell membrane with it. (6) The viral particles seek out new host cells to infect, spreading the virus through the system and starting the process anew. Figure #1 shows a graphical representation of the HIV life cycle.
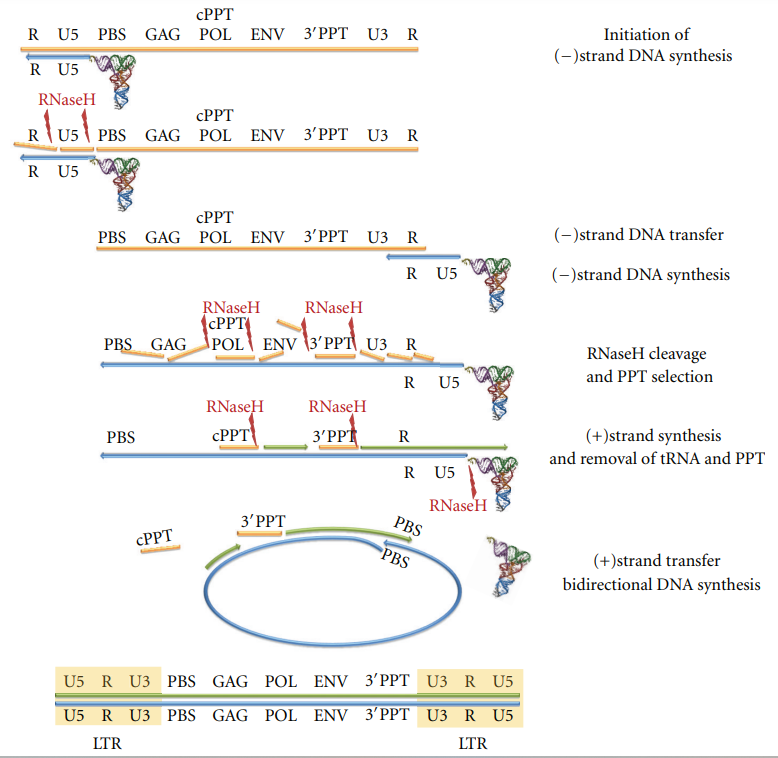
The actual process of HIV reverse transcription begins when the host cell tRNALsy3 hybridizes at the 5’-end of the (+) strand on the RNA genome. tRNALys3 is then used as a primer for the (-) strand DNA synthesis. DNA synthesis continues until the 5’ end of the RNA strand. The next step in the process involves RNase hydrolysis of the RNA/DNA hybrid to determine the (-) strand strong stop DNA by exposing the ssDNA product. The (-) strand DNA is then lengthened further and hybridized with the R region at the 3’-end of the ssRNA strand to transfer it. DNA synthesis continues when the RNase H function cleaves the RNA strand in the RNA/DNA hybrid at multiple different points, leaving two specific sections undamaged. These sections are cPPT and 3’PPt, both of which are resistant to the RNase H cleavage. DNA synthesis for the (-) strand is initiated again, only this time using the PPT strand fragments as primers. Hydrolysis of the PPT segments and the junction of the tRNA/DNA hybrid is then initiated by the RNase H, which frees up the PBS sequence of the (+) strand DNA. The PBS sequence from the (+) strand of DNA then anneals to the PBS on the (-) strand DNA. The DNA synthesis then continues, using strand displacement synthesis to get a linear dsDNA with long terminal repeats on both ends as the product (4). Figure #2 shows a graphical representation of reverse transcription.
DiseaseHIV-1 is a bloodborne and sexually transmitted infection in humans. Upon entry to the body, the HIV-1 virus infects white blood cells, or helper T cells. This results in the production of more virus, and eventually cell death and AIDS using the process of reverse transcription (1). First, the viron performs endocytosis to enter the host cell. Once in the cell, the viron's uncoats to release the viral RNA and HIV-1 RT. The HIV-1 RT is then able to make proviral DNA from the viral RNA (this is known as reverse transcription), and other enzymes integrate the proviral DNA into the host cells genome within the nucleus. Normal cell transcription then produces mRNA, and facilitates the construction of thousands of new virons, which are then released from the host cell. Over time, the immune system is essentially destroyed, which consequently causes Acquired Immunodeficiency Syndrome, or AIDS. People who develop AIDS begin to become very ill with fever, a sore throat, and a rash, since the body can no longer actively fight pathogens (1). RelevanceThe finding of HIV-1 RT was monumental for offering treatment to individuals living with HIV-1. After its discovery, the development of nucleoside reverse transcriptase inhibitors (NRTIs) and non-nucleoside reverse transcriptase inhibitors (NNRTIs), which are responsible for inhibition of different mechanisms of reverse transcription. Once the NNRTs come in contact with the RT, the process is stoped and the proviral DNA remains incomplete (7). The first drug approved to treat HIV was a NRTI, or a reverse transcriptase inhibitor called Zidovudine (AZT), and it was released in 1987 (7). Additionally, the are 13 more approved drugs to treat HIV that inhibit RT, and this is out of 26 approved drugs total (5). So, the discovery of reverse transcriptase was imperative to providing treatment to millions of HIV+ patients. Structural highlightsHIV reverse transcriptase is an asymmetric heterodimer of a homodimer. There are 560 residues in shown in blue, and 440 residues in Chain B, shown in green (3). In both chains, Alpha helices and Beta sheets can be found in both chains. DNA polymerase, connection, and RNase H domains are present in chain A ,p66, as well as active sites for DNA polymerase and RNase H (9). The connection domain is a protease-sensitive region that is involved in intersubunit interactions (10). Additionally, this domain connects the DNA polymerase domain and the RNase H domain. DNA polymerase consists of three subdomains, which are described as the fingers, palms, and thumbs. Protease-mediated cleavage of the RNase H domain of p66 produces chain B, p51. The DNA polymerase and connection domains of p51 differ a lot compared to p66. In p66, the conformation is "open" in order to accommodate substrates, while in p51, the conformation is "closed" and it plays predominantly a structural role (9). References(1) Morier, D. Reverse transcriptase. https://www.britannica.com/science/reverse-transcriptase (accessed Nov 14, 2022). (2) Kati, W.M.; Johnson, K.A.; Jerver, L.F.; Anderson, K.F. Mechanism and Fidelity of HIV Reverse Transcriptase. J. Biol. Chem. 1992, 267(36), 25988-25997 (3) Abbondanzieri, E. A.; Bokinsky, G.; Rausch, J. W.; Zhang, J. X.; Le Grice, S. F.; Zhuang, X. Dynamic Binding Orientations Direct Activity of HIV Reverse Transcriptase. Nat. 2008, 453(7192), 184–189. (4) Esposito, F.; Corona, A.; Tramontano, E. HIV-1 Reverse Transcriptase Still Remains a New Drug Target: Structure, Function, Classical Inhibitors, and New Inhibitors with Innovative Mechanisms of Actions. Mol. Biol. Int. 2012, 2012, 1–23. (5) Hu, W. S.; Hughes, S. HIV-1 Reverse Transcription. Cold Spring Harb. Prospect. Med. 2012 2(10) doi: 10.1101/cshperspect.a006882 (6) Sluis-Cremer, N.; Tachedjian, G. Mechanisms of Inhibition of HIV Replication by Non-Nucleoside Reverse Transcriptase Inhibitors. Virus Res. 2008, 134 (1-2), 147–156. (7) Singh, A. K.; Das, K. Insights into HIV-1 Reverse Transcriptase (RT) Inhibition and Drug Resistance from Thirty Years of Structural Studies. Viruses 2022, 14 (5), 1027. (8) Jacobo-Molina, A.; Arnold, E. Perspectives in Biochemistry: HIV Reverse Transcriptase Structure-Function Relationships. Biochem. 1991, 30 (26), 6351-6361. (9) Sluis-Cremer N.; Retroviral reverse transcriptase: Structure, function and inhibition. Enzymes [Online], 2021 Jul 24, p 179-194. Medline. https://www.nlm.nih.gov/medline/ (accessed Oct. 21, 2022) (10) Lowe, D.M.; Aitken, A.; Bradley, C.; Darby, G.K.; Larder, B.A.; Powell, K.L.; Purifoy D.J.M.; Tisdale, M.; Stammers, D.K. Accelerated Publications: HIV-1 Reverse Transcriptase: Crystallization and Analysis of Domain Structure by Limited Proteolysis. Biochem. 1988, 27, 8884-8889 | ||||||||||||