This is a default text for your page '. Click above on edit this page' to modify. Be careful with the < and > signs.
You may include any references to papers as in: the use of JSmol in Proteopedia [1] or to the article describing Jmol [2] to the rescue.
Function
Structure
Overview
The protein combines 3 separate (see notes for more). SHOC2, PP1C, and MRAS, to form the active protein (SMP complex), as seen in Figure 2[3]. The SMP complex was determined via cryo-electron microscopy as well as x-ray diffraction. The overall structure has PP1C and MRAS bound within the concave surface of SHOC2, leaving the catalytic site of PP1C and the GTP binding cleft in MRAS exposed.
SHOC2
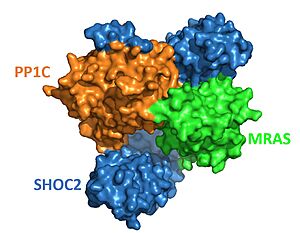
Figure 2. Surface representation of SHOC2-PP1C-MRAS (
PDB 7upi). SHOC2 (blue), PP1C (orange) and MRAS (green).
SHOC2 is a scaffolding protein which acts as a cradle to bind PP1C and MRAS (REF), serving as an aggregation point for these 3 signaling proteins. is a leucine rich repeat (LRR) protein consisting of 20 consecutive . LRR motifs form an an extended BETA sheet on the inner concave surface of SHOC2 with alpha helices facing outward, which help bind the whole protein. These LRR motifs result in a largely hydrophobic core within the concaved region.[4].
PP1C
is the catalytic domain of the phosphatase enzyme PP1, which removes reversible phosphorylations from signaling proteins. PP1C is a serine/threonine phosphatase that is involved in cellular signaling pathways that control cell growth, division, and metabolism (REF) and explain more, see notes.
MRAS
is a monomeric GTPase and is anchored in the cell membrane. When MRAS binds GTP, it becomes active, and the rest can only bind when this is activated[3]. MRAS is a subvariant of the RAS protein and therefore shares most of it's regulatory and effector interactions[5]. While other RAS variants bind in complex with SHOC2 and PP1 to allow it to have phosphatase activity, MRAS binds the tightest.
Stabilizing Interactions
exclusively through its concave LRRs[6], primarily by the descending loop and strands of LRR domains 2-10. with the ascending loops of the SHOC2 LRR regions, and is further engaged through the N-terminal region of SHOC2 containing the [7]. The initial forming of the complex begins with SHOC2-PP1C engagement, then is completed and stabilized by the GTP-loaded MRAS binding (apparently described differently elsewhere?) [7]. Once associated with SHOC2, . Binding to MRAS localizes the other two proteins to the RAS signaling regions of the membrane to begin cellular signaling.[6].
Implications
The SHOC2-MRAS-PP1C complex's key role in the regulation of the MAPK-RAF pathway means that minor changes in its structure or function can have drastic biological consequences. Unregulated activation of the MAPK pathway is one of the most common causes of human cancer due to unchecked cell division and proliferation (REF). Mutations that stabilize the interactions of the SMP complex enhances PP1C phosphatase activity [7], leading to increased RAF signaling and accelerated cell division.
The unregulated MAPK pathway is also responsible for a multitude of developmental disorders commonly known as RASopathies [8]. One such RASopathy caused by the mutation of a RAF kinase is known as Noonan Syndrome (NS), which enhances complex formation (WHY). NS is a genetic disorder that can prevent normal development in different parts of the body in a variety of ways. NS patients often have unusual facial characteristics, short stature, a variety of heart defects and disease, and other physical problems and developmental delays.
SHOC2
SHOC2 is a domain which acts as a cradle to bind PP1C and MRAS. The
is a leucine rich repeat (LRR) protein that consists of 20 consecutive
. This motif results in an extended beta sheet on the inner concavity of the protein surface with alpha helices facing outward. This results in a largely hydrophobic core.
protein phosphatase 1 catalytic subunit ( ) is highly characterized of serine/ threonine phosphatase. PP1C
>
associates with the ascending loops of the SHOC2 LRR regions.
MRAS
binds to SHOC2 primarily by the descending loop and strands of each LRR domains 2-10. Once associated with SHOC2, it binds to PP1C and guides the holoenzyme complex to the cell membrane to begin signaling.
PP1C
Interactions
This is a sample scene created with SAT to by Group, and another to make of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes.

