Sandbox Reserved 1788
From Proteopedia
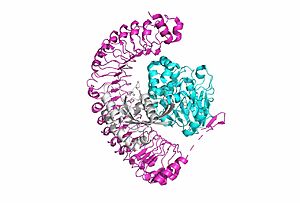
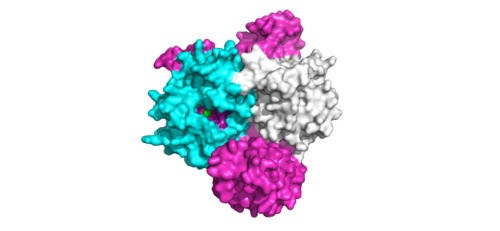
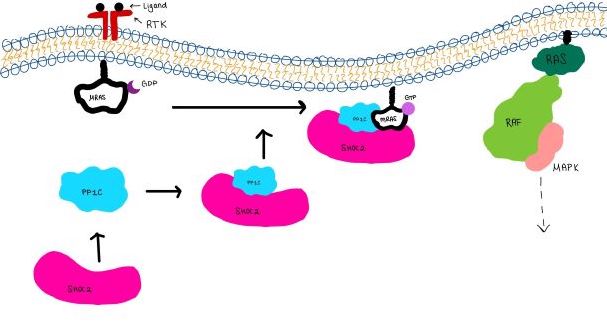
SHOC2-PP1C-MRASIntroduction(SMP) is a ternary holoposphatase complex formed by the individual proteins: SHOC2, PP1C, and MRAS. Formation of this complex begins with a signal binding to a receptor tyrosine kinase receptor(RTK). This causes membrane-bound MRAS to exchange GDP for GTP. From here the complex comes together in the plasma membrane. Its role in MAPK signaling is the dephosphorylation of the N-terminal phosphoserine (NTpS) on the RAF complex leading to further downstream signaling effects. Overall StructureSHOC2is a crescent-shaped scaffold protein that is composed of 20 leucine-rich repeat domains that form a solenoid structure. The leucine-rich region forms a concave hydrophobic core which is necessary for binding with PP1C and MRAS. SHOC2 is the crucial mediator for SHOC2-PP1C-MRAS complex formation. PP1C is a catalytic protein. After forming a ternary complex, the on the protein interacts with Raf to act as a phosphatase and dephosphorylate Ser 259. PP1C's active site is adjacent to a hydrophobic patch. It's theorized that the hydrophobic patch binds to the C-terminal of NTpS on RAF, the target for dephosphorylation. PP1C can act as a phosphatase in the absence of SHOC2 but PP1C lasks intrinsic substrate selectively. So SMP complex formation is necessary for PP1C specificity to RAF.
MRASis a membrane-bound structure that aids the complex in localizing near other structures such as the RAS-RAF-MAPK complex in order to initiate downstream signaling. In its inactive state, MRAS is bound to GDP. When signaled by growth factors, the GDP is exchanged for GTP. The now undergoes a conformational change of the . This conformational change activates the protein allowing it to bind with the SHOC2-PP1C complex. Without the conformational change when GDP is exchanged to GTP, the GDP-MRAS wouldn't be able to bind to SHOC2 because of steric clashing. In comparison to other RAS proteins, MRAS has a greater affinity for the SHOC2-PP1C complex. MRAS engages the SHOC2-PP1C complex and RAF on the same surface indicating that for RAF signaling two separate active MRASs are needed. Having two MRASs also help with the co-localization of PP1C to the NTpS region on RAF.
Key Ligand InteractionsSHOC2 and PP1Con its leucine rich region(LRR). Specifically, on two broad surfaces between LRR2 and LRR5 and between LRR7 and LRR11. Mutations made to the LRR were shown to completely inhibit the binding of PP1C. Five main are made: E56-R182, E167-R203, E54-K180, R187-H178, R188-E155. The binding regions can also be shown as acidic and basic patches on and . The corresponding patches interact to form a . These interactions do not result in significant conformational changes.
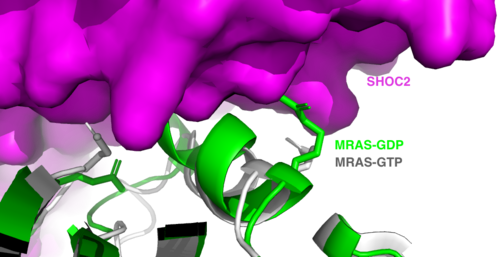
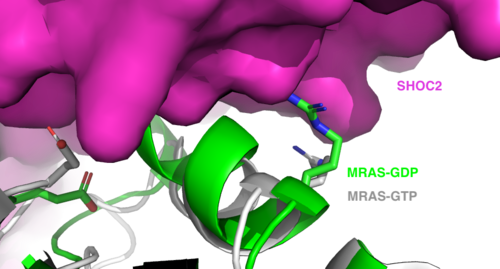
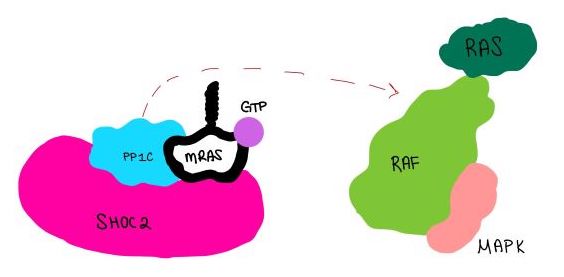
SHOC2 and MRASMRAS is initially bound to GDP causing it to be in its inactive state. This form cannot bind to the SHOC2-PP1C complex due to steric clashing (Figure 2). Once GDP is exchanged for GTP to activate the protein, occur within the switch I and switch II regions to allow . These include hydrogen bonds and pi stacking. The primary hydrogen bonds are R288-Q71 and R177-E47. Pi staking occurs at R104-R83. PP1C and MRASThe interactions between PP1C and MRAS are mediated by four main hydrogen bonds: R188-D48, M190-Q35, D197-H53, Q198-K36. It is unclear whether PP1C must bind to SHOC2 before MRAS binds or if PP1C and MRAS can bind to SHOC2 at the same time. Signaling PathwayThe SMP signaling pathway begins with the formation of the SMP complex. Initially, a ligand must bind to a receptor tyrosine kinase. This signals SHOC2 to bind to PP1C forming a binary complex that then binds to the membrane bound MRAS. Some literature indicates that the three proteins bind at the same time but the order is largely unknown. Figure 2 shows the proteins binding one at a time. Once the SMP complex forms, its target is the N-terminal phosphoserine (NTpS) also known as S259. The serine is directly dephosphorylated by PP1C by SHOC2 and MRAS increase its specificity for S259. Mutations affecting SMP complex formation and stability have been shown to increase or decrease MAPK signaling. Increased stability of the complex increases MAPK signaling while decreased stability decreases signaling.
Disease RelevanceRASopathiesRASopathy is a broad term used to describe developmental syndromes that stem from germline mutations of proteins along the RAS/MAPK pathway such as SHOC2, PP1C, and MRAS. These mutations can be either gain or loss of function. Rasopathies can also lead to cancer. CancerBecause the RAS/MAPK pathway activated by SMP regulates cell proliferation and survival, overactivity can cause tumor formation and cancer. For example, the complex has been found to play a role in the perpetuation of melanoma, leukemia, and lung cancer. Future StudiesFurther study of the SMP complex includes clarification of the steps of the pathway. Firstly, the order of binding to form the SMP complex is unclear. Furthermore, the interaction between SMP and the Raf complex is largely unknown. Study into this step is especially important to understand how SMP activates downstream signaling. The current knowledge of SMP can be used to study possible treatments for rasopathies and cancer. For example, the development of inhibitors that target SMP binding could prevent the effects caused by mutations that overactivate SMP. Another possible point of inhibition is the growth factor that signals SHOC2-PP1C and Raf to the cell membrane.
References
Student ContributorsMadeline Gilbert Inaya Patel Rushda Hussein | ||||||||||||