Sandbox Reserved 347
From Proteopedia
| This Sandbox is Reserved from January 10, 2010, through April 10, 2011 for use in BCMB 307-Proteins course taught by Andrea Gorrell at the University of Northern British Columbia, Prince George, BC, Canada. |
To get started:
More help: Help:Editing |
| |||||||||
| 2iko, resolution 1.90Å () | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ligands: | |||||||||
| Gene: | REN (Homo sapiens) | ||||||||
| Activity: | Renin, with EC number 3.4.23.15 | ||||||||
| |||||||||
| |||||||||
| Resources: | FirstGlance, OCA, RCSB, PDBsum | ||||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||||
Contents |
Introduction
(pronounced /ˈriːnɨn/ REE-nin) is also known as angiotensinogenase, a monospecific enzyme that participates in the body's renin-angiotensin system (RAS). Renin is responsible for catalyzing the rate-limiting step in the synthesis of angiotensin II. Once renin and pro-renin bind to the pro-renin receptor, there is an increased enzymatic activity and additional physiological effects. [1]
Structure
Renin belongs in a family called aspartic proteases which use an aspartate residue for the catalysis of their peptide substrate. X-ray diffraction experiments has shown there is a striking similarity among the structures of aspartyl proteases. [2] Renin consists of two homologous lobes each containing an aspartic acid. Between the lobes is the active site, which is catalyzed by the aspartic acid residues, a characteristic trait of all aspartate proteases. [1] Renin in its full mature form has a mass of 37 kDa and contains 340 amino acids.[3] Uniquely from other proteases, Renin has two β-carboxyl groups of Asp32 and Asp215 which protrude from each of the two lobes into the active site and has a large flap covering the .[4]
|
Function
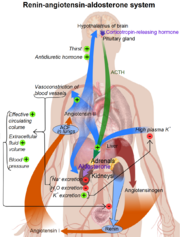
Renin plays a key role in the Renin-Angiotension sysmtem (RAS). It is essential in facilitating the conversion of angiotension to angiotension II, which is the active component of the RAS system.[5] This system is responsible for the regulation of blood pressure, stimulation of the secretion of aldosterone which effects the salt and water balance.[5]
- Angiotensinogen is released into the bloodstream by the liver.
- Likewise, Renin is secreted by the kidneys into the bloodstream where is meets with angiotensinogen.
- Once united, angiotensinogen form the decapeptide angiotensin (ANG) I.
- ANG I is then activated by Angiotensin converting enzyme (ACE) to form the octapeptide ANG II.
- ANG II then acts on specific receptors such as ones responsible for vasoconstriction or the release of aldosterone from the adrenal cortex. [6]
|
References
- ↑ 1.0 1.1 Gradman AH, Kad R. Renin inhibition in hypertension. J Am Coll Cardiol. 2008 Feb 5;51(5):519-28. PMID:18237679 doi:10.1016/j.jacc.2007.10.027
- ↑ K Akahane, H Umeyama, S Nakagawa, I Moriguchi, S Hirose, K Iizuka, and K Murakami. "Three-dimensional structure of human renin". Hypertension. 1985;7:3-12
- ↑ Armstrong C. The vision of the pore. Science. 1998 Apr 3;280(5360):56-7. PMID:9556453
- ↑ Inagami T. Structure and function of renin. J Hypertens Suppl. 1989 Apr;7(2):S3-8. PMID:2666611
- ↑ 5.0 5.1 Reid IA, Morris BJ, Ganong WF. The renin-angiotensin system. Annu Rev Physiol. 1978;40:377-410. PMID:205167 doi:http://dx.doi.org/10.1146/annurev.ph.40.030178.002113
- ↑ Paul M, Poyan Mehr A, Kreutz R. Physiology of local renin-angiotensin systems. Physiol Rev. 2006 Jul;86(3):747-803. PMID:16816138 doi:10.1152/physrev.00036.2005